5-methyl-4-[1]naphthyl-thiazol-2-ylamine | 107411-05-2
中文名称
——
中文别名
——
英文名称
5-methyl-4-[1]naphthyl-thiazol-2-ylamine
英文别名
2-Thiazolamine, 5-methyl-4-(1-naphthalenyl)-;5-methyl-4-naphthalen-1-yl-1,3-thiazol-2-amine
CAS
107411-05-2
化学式
C14H12N2S
mdl
MFCD20171325
分子量
240.329
InChiKey
WOXJGUKYQNONEE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
反应信息
-
作为产物:描述:2-bromo-1-(1-naphthyl)-1-propanone 、 硫脲 在 甲醇 、 盐酸 、 乙醚 、 二氯甲烷 、 氯仿 、 水 、 Sodium sulfate-III 作用下, 以 甲苯 为溶剂, 反应 2.0h, 以The title compound (1) (4.45 g, m.p. 194-195° C.) was obtained的产率得到5-methyl-4-[1]naphthyl-thiazol-2-ylamine参考文献:名称:5-Ht2b receptor antagonists摘要:本发明涉及公式(I)的化合物:其中R1选自H,及可选取的取代C1-6烷基,C3-7环烷基,C3-7环烷基-C1-4烷基和苯基-C1-4烷基;R2和R3是:(i)独立选自H,R,R',SO2R,C(═O)R,(CH2)nNR5R6,其中n为1至4,R5和R6独立选自H和R,其中R为可选取的取代C1-4烷基,R'为可选取的取代苯基-C1-4烷基,或(ii)与它们所连接的氮原子一起形成可选取的取代C5-7杂环基;R4为可选取的取代C9-14芳基基团;其用途为制成药物,特别是用于治疗通过5-HT2B受体拮抗缓解的疾病。公开号:US20050154031A1
文献信息
-
5-HT2B RECEPTOR ANTAGONISTS申请人:Pharmagene Laboratories Ltd公开号:EP1474141A1公开(公告)日:2004-11-10
-
[EN] 5-HT2B RECEPTOR ANTAGONISTS<br/>[FR] ANTAGONISTES DU RECEPTEUR 5-HT2B申请人:PHARMAGENE LAB LTD公开号:WO2003068227A1公开(公告)日:2003-08-21The present invention concerns compounds of formula (I): wherein R1 is selected from the group consisting of H, and optionally substituted C 1-6 alkyl, C 3-7 cycloalkyl, C 3-7 cycloalkyl-C 1-4 alkyl, and phenyl-C 1-4 alkyl;R2 and R3 are either: (i) independently selected from H, R, R', SO2R, C(=O)R, (CH2)nNR5R6, where n is from 1 to 4 and R5 and R6 are independently selected from H and R, where R is optionally substituted C 1-4 alkyl group, and R' is an optionally substituted phenyl- C 1-4 alkyl group, or (ii) together with the nitrogen atom to which they are attached, form an optionally substituted C 5-7 heterocyclic group; R4 is an optionally substituted C 9-14 aryl group; their use as pharmaceuticals, in particular for treating conditions alleviated by antagonism of a 5-HT 2B receptor.
表征谱图
-
氢谱1HNMR
-
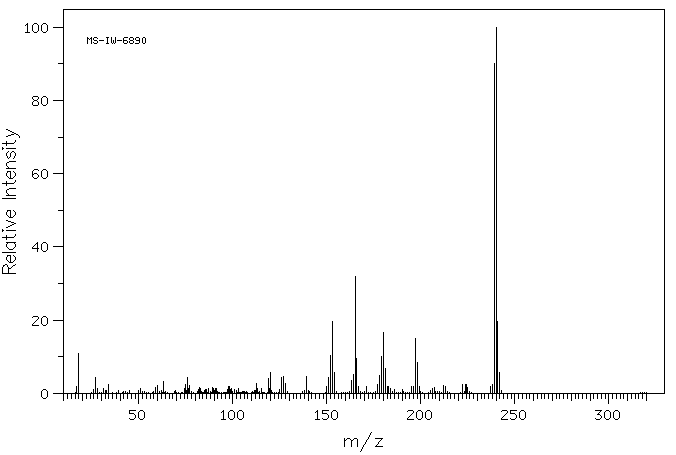
质谱MS
-
碳谱13CNMR
-
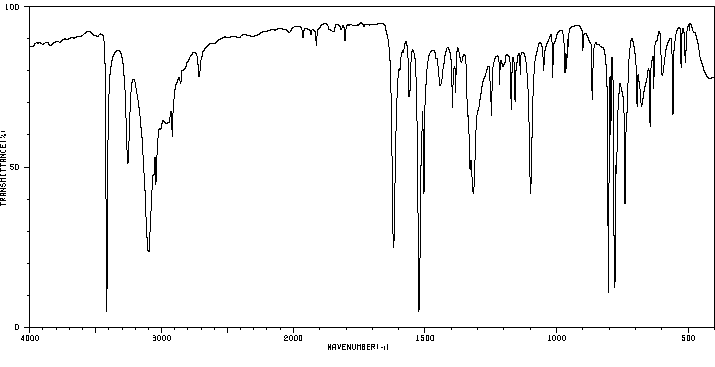
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮