N,N'-二乙基-N,N'-二亚硝基乙二胺 | 7346-14-7
中文名称
N,N'-二乙基-N,N'-二亚硝基乙二胺
中文别名
——
英文名称
3,7-Dinitroso-3,7-diazaoctan
英文别名
Ethylenediamine, N,N'-diethyl-N,N'-dinitroso-;N-ethyl-N-[2-[ethyl(nitroso)amino]ethyl]nitrous amide
CAS
7346-14-7
化学式
C6H14N4O2
mdl
——
分子量
174.203
InChiKey
OZTCCANDHYUXMW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:305.18°C (rough estimate)
-
密度:1.2297 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:65.3
-
氢给体数:0
-
氢受体数:6
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Gol'din,G.S. et al., Journal of Organic Chemistry USSR (English Translation), 1974, vol. 10, p. 1644 - 1648摘要:DOI:
-
作为产物:描述:参考文献:名称:Gol'din,G.S. et al., Journal of Organic Chemistry USSR (English Translation), 1970, vol. 6, p. 1177 - 1179摘要:DOI:
文献信息
-
Nitric oxide reactivity of copper(ii) complexes of bidentate amine ligands: effect of substitution on ligand nitrosation作者:Moushumi Sarma、Biplab MondalDOI:10.1039/c2dt11082b日期:——Three copper(II) complexes with bidentate ligands L1, L2 and L3 [L1, N,N/-dimethylethylenediamine; L2, N,N/-diethylethylenediamine and L3, N,N/-diisobutylethylenediamine], respectively, were synthesized as their perchlorate salts. The single crystal structures for all the complexes were determined. The nitric oxide reactivity of the complexes was studied in acetonitrile solvent. The formation of thermally
-
21-Hydroxycorticosteroid esters, and compositions containing them申请人:THE UPJOHN COMPANY公开号:EP0106453A1公开(公告)日:1984-04-2521-Hydroxycorticosteroid esters which can have good solution stability and are therefore suitable for formulation into aqueous compositions for injection or infusion, have the formula wherein St is the corticosteroid without its 21-hydroxy group; Y is a bond. -O- or -S-; Xis -CO-NR-, -NR-CO-, -O-, -S-, -SO- or-S02- and R is hydrogen or C1-4 alkyl; m is an integer of from 1 to 5 and n is an integer of from 2 to 9, provided that m + n ≤ 10; and either R, and R2 are each C1-4 alkyl optionally substituted by one hydroxyl group or -NR1R2 is a monocyclic heterocyclic group; or a salt thereof.
-
US4456602A申请人:——公开号:US4456602A公开(公告)日:1984-06-26
表征谱图
-
氢谱1HNMR
-
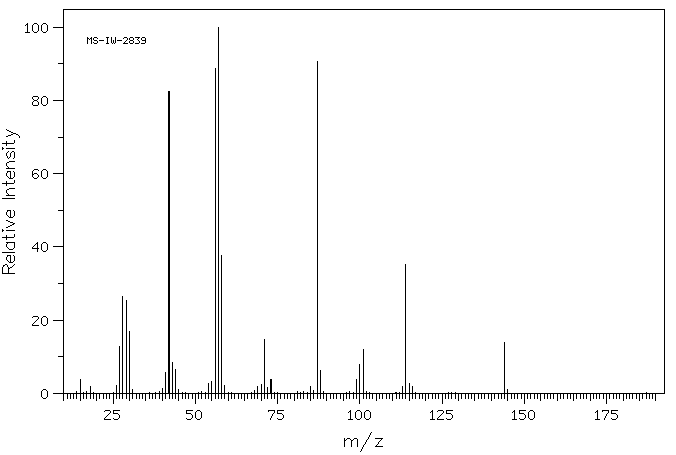
质谱MS
-
碳谱13CNMR
-
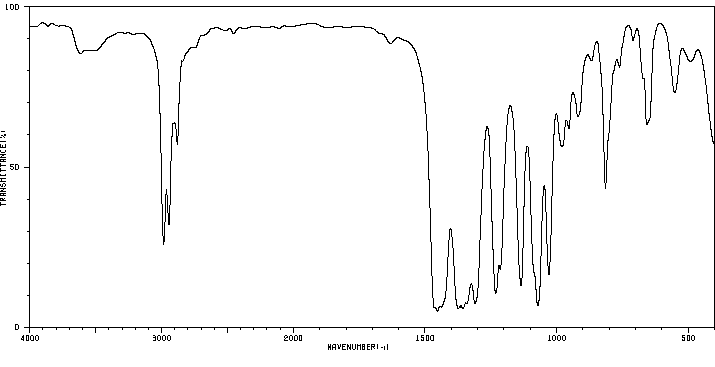
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷