trans-1,4-Diacetamido-cyclohexan | 2077-92-1
中文名称
——
中文别名
——
英文名称
trans-1,4-Diacetamido-cyclohexan
英文别名
trans-N.N'-Diacetyl-1.4-diamino-cyclohexan;trans-1.4-Bis--cyclohexan;N,N'-(trans-cyclohexane-1,4-diyl)-bis-acetamide; trans-1.4-bis-acetylamino-cyclohexane;N,N'-(trans-Cyclohexan-1,4-diyl)-bis-acetamid; trans-1.4-Bis-acetamino-cyclohexan;trans-1.4-Bis-acetamino-cyclohexan
CAS
2077-92-1
化学式
C10H18N2O2
mdl
——
分子量
198.265
InChiKey
TVILGUBKPIUIFC-MGCOHNPYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.57
-
重原子数:14.0
-
可旋转键数:2.0
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:58.2
-
氢给体数:2.0
-
氢受体数:2.0
反应信息
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 生成 trans-1,4-Diacetamido-cyclohexan参考文献:名称:Curtius, Journal fuer Praktische Chemie (Leipzig), 1915, vol. <2>91, p. 36摘要:DOI:
文献信息
-
Organic Compounds申请人:Collingwood Stephen Paul公开号:US20080312217A1公开(公告)日:2008-12-18A Compound of formula (I) or tautomers, or stereoisomers, or solvates, or pharmaceutically acceptable salts thereof, wherein M 1 , M 1 , L 1 , L 2 , W 1 , W 2 , X 1 , X 2 , Y 1 , Y 2 , A, R 5 , and R 5a are as defined herein for the treatment of conditions mediated by the blockade of an epithelial sodium channel, particularly an inflammatory or allergic condition.
-
Synthesis of analogs of N-(2-chloroethyl)-N'-(trans-4-methylcyclohexyl)-N-nitrosourea for evaluation as anticancer agents作者:Thomas P. Johnston、George S. McCaleb、Sarah D. Clayton、Jerry L. Frye、Charles A. Krauth、John A. MontgomeryDOI:10.1021/jm00212a019日期:1977.2The superior activity of N-(2-chloroethyl)-N'-(trans-4-methylcyclohexyl)-N-nitrosourea (MeCCNU) against advanced murine Lewis lung carcinoma in comparisons with the cis form and other nitrosoureas prompted the synthesis of a number of MeCCNU analogues, including several cis-trans pairs. The methyl group was replaced by a variety of substituents (CO2H, CH2CO2H, CO2Me, CH2OAc, CH2Cl, OMe); the trans-3-methylcyclohexyl, cis-2-methyl-1,3-dithian-5-yl, cis- and trans-2-methyl-1,3-dithian-5-yl-tetraoxide, and 1-methylhexyl (open-chain) analogues were also prepared. Preliminary tests against murine leukemia L1210 revealed therapeutic indices (ED50/LD10) ranging from 0.26 to 0.79; all but three analogues effected 50% cure rates at nontoxic doses, the open-chain analogue being one of the least active. In terms of therapeutic index, diequatorial (trans-4) isomers were, with one exception, as active as or, in four of the eight examples, somewhat more active than the corresponding axial-equatorial (cis-4) isomers. In this series, four of the five 2-fluoroethyl analogues prepared were clearly inferior to the corresponding 2-chloroethyl analogues.
-
US7803804B2申请人:——公开号:US7803804B2公开(公告)日:2010-09-28
表征谱图
-
氢谱1HNMR
-
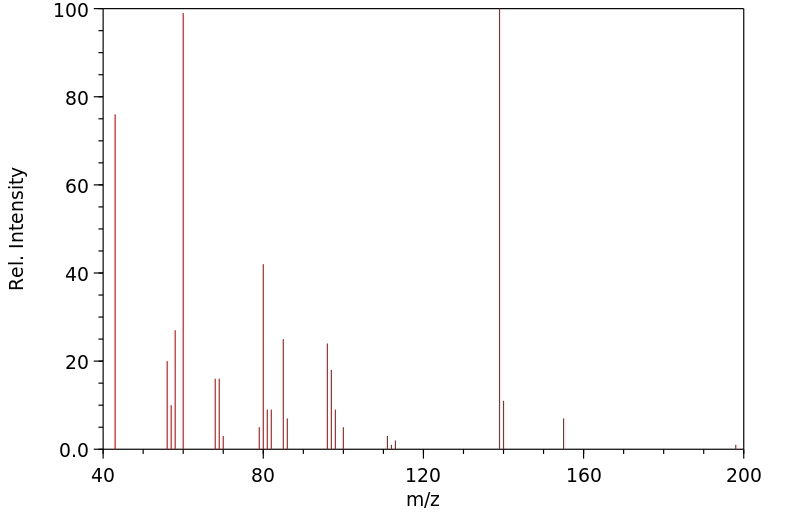
质谱MS
-
碳谱13CNMR
-
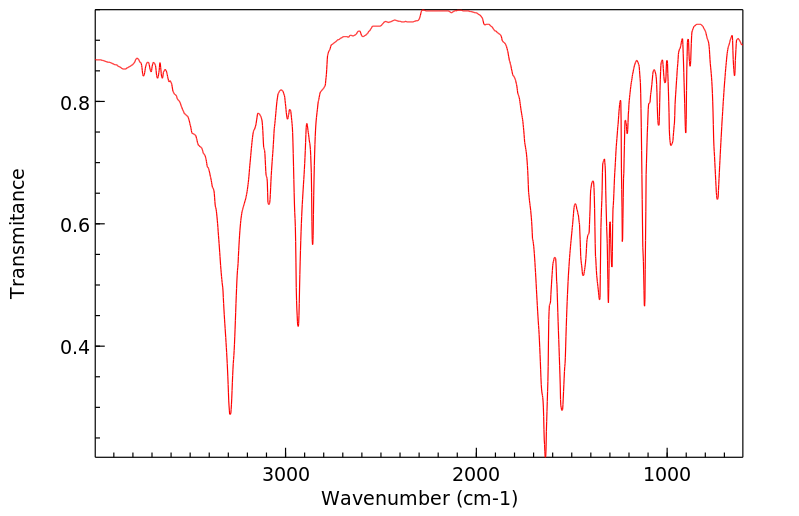
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
辛二亚氨酸二甲酯二盐酸盐
辛二亚氨酸二甲酯
脲鎓溴化物
羟甲基脲
羟基脲
缩二脲
缩三脲
碳亚胺酸二丙酯
硬脂酰胺
癸醯胺
甲酰胺-d3
甲酰胺
甲基氰基脒醚
甲基双环[2.2.1]庚-5-烯-2-甲亚氨酸酯
甲基乙酰亚胺酯盐酸盐
甲基N-氰基-N'-甲基氨基亚胺酸酯
甲基N-异丙基-N-甲基氨基亚胺酸酯
甲基3-氯代丙酸乙酯盐酸盐
甲基2-氯乙亚氨酸酯盐酸盐
甲基2,2-二乙氧基乙亚氨酸酯
甲基(3-甲基-1-硫基-3-丁烯-2-基)氨基甲酸酯
甲亚胺异丙酯 盐酸盐
甲亚胺乙酯盐酸盐
甘氨酰胺
环戊烷甲亚氨酸乙酯
环丙酰胺
环丙烷甲亚胺酸乙酯
溴米索伐
涕灭威
氰基甲酯
氰基亚氨代甲酸甲酯
氰基乙酯
氨基甲酸乙酯
氨基丙酮缩氨基脲盐酸盐
氟乙酰胺
戊亚氨酸甲酯
异丙基氨基亚胺酸酯盐酸盐(1:1)
庚二亚氨酸二甲酯二盐酸盐
叔丁基三氯乙酰亚胺酯
十六酰胺乙醇
亚油酰胺
亚氨酰乙酸甲酯
亚氨戊酸甲酯盐酸盐
亚氨基碳酸二甲酯
二硫代二丙亚氨酸二甲酯
二乙氧基甲亚胺
二乙基丙烷二亚氨酸酯二盐酸盐
乙酰胺
乙炔二羰酰胺
乙氧亚氨基乙酸乙酯