5-氯苯并呋喃 | 17348-69-5
中文名称
5-氯苯并呋喃
中文别名
5-氯苯并呋噁
英文名称
5-chlorobenzofurazan-1-oxide
英文别名
5-chlorobenzofuroxan;5-Chlorbenzofuroxan;5-Chlorobenzofurazan 1-oxide;5-chloro-1-oxido-2,1,3-benzoxadiazol-1-ium
CAS
17348-69-5
化学式
C6H3ClN2O2
mdl
MFCD00068063
分子量
170.555
InChiKey
DHPQXIQZZCNOLI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:45-48°C
-
沸点:287.1±32.0 °C(Predicted)
-
密度:1.61 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
危险类别码:R22
-
海关编码:2934999090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:潜在的抗白血病和免疫抑制药物。一些苯并-2,1,3-恶二唑(苯并呋喃)及其N-氧化物(苯并呋喃)的制备及其体外药理活性。摘要:DOI:10.1021/jm00308a027
-
作为产物:描述:N-(4-氯-2-硝基苯基)乙酰胺 在 盐酸 、 sodium azide 、 硫酸 、 sodium acetate 、 sodium nitrite 作用下, 以 甲苯 为溶剂, 反应 2.5h, 生成 5-氯苯并呋喃参考文献:名称:Hypoxia-Selective Agents Derived from Quinoxaline 1,4-Di-N-oxides摘要:Hypoxic cells, which are a common feature of solid tumors, but not normal tissues, are resistant to both anticancer drugs and radiation therapy. Thus the identification of drugs with selective toxicity toward hypoxic cells is an important objective in anticancer chemotherapy. The benzotriazine di-N-oxide (SR 4233, Tirapazamine) has been shown to be an efficient and selective cytotoxin for hypoxic cells. Since the bioreductive activation of Tirapazamine is thought to be due to the presence of the 1,4-di-N-oxide moiety, a series of 3-aminoquinoxaline-2-carbonitrile 1,4-di-N-oxides with a range of electron-donating and -withdrawing substituents in the 6- and/or 7- positions has been synthesized and evaluated for toxicity to hypoxic cells. Electrochemical studies of the quinoxaline di-N-oxides and Tirapazamine showed that as the electron-withdrawing nature of the 6(7)-substituent increases, the reduction potential becomes more positive and the compound is more readily reduced. Apart from the unsubstituted 6a and the 6,7-dimethyl derivative 6c, the quinoxaline di-N-oxides have reduction potentials significantly more positive than Tirapazamine (E(pc)-0.90 V). The most potent cytotoxins to cells in culture were the 6,7,-dichloro and 6,7-difluoro derivatives 6i and 6l, which were 30-fold more potent than Tirapazamine. The 6(7)-fluoro and 6(7)-chloro compounds, 6e and 6h, showed the greatest hypoxia selectivity. Four of the compounds, 6e, 6f, 6h and 6i, killed the inner cells of multicellular tumor spheroids in vitro. In vivo Balb/c mice tolerated a dose of these four compounds twice the size of that of Tirapazamine. This study demonstrates that quinoxaline 1,4-di-N-oxides could provide useful hypoxia-selective therapeutic agents.DOI:10.1021/jm00010a023
文献信息
-
Ammonium Sulphate—Magnesium Promoted Selective Reduction of Aromatic Nitro Compounds作者:Dipak Prajapati、Harsha N. Borah、Jagir S. Sandhu、Anil C. GhoshDOI:10.1080/00397919508011478日期:1995.12Abstract Various nitroarenes and 2,1,3-benznooxadiazole-1-oxides were selectively and rapidly reduces to their corresponding amino and diamino compounds respectively in high yields using (NH4SO4-Mg/A1/Bi, a new reduction system.
-
Quinoxaline derivatives申请人:Research Corporation公开号:US04343942A1公开(公告)日:1982-08-10The synthesis of quinoxaline and benzimidazole-N-oxides and of ester and amide derivatives of 3-hydroxy-2-quinoxalinecarboxylic acid by a novel process consisting of the reaction between a benzofuroxan and an activated methylene-containing compound under basic conditions.
-
Cu-Catalyzed π-Core Evolution of Benzoxadiazoles with Diaryliodonium Salts for Regioselective Synthesis of Phenazine Scaffolds作者:Jinyu Sheng、Ru He、Jie Xue、Chao Wu、Juan Qiao、Chao ChenDOI:10.1021/acs.orglett.8b01748日期:2018.8.3The Cu-catalyzed regioselective synthesis of phenazine N-oxides was realized from benzoxadiazoles and diaryliodonium salts. The process was initiated by the electrophilic arylation of benzoxadiazoles with diaryliodonium salts and followed by benzocyclization reactions. The further reduction of N-oxides in situ to phenazine scaffolds and deviation to organic fluorescent materials were readily accomplished
-
4-Cyano-2-oxo-1,2,4-oxadiazolo[2,3-<i>a</i>]quinoxaline 5-<i>N</i>-oxides. New synthetic method and reaction with alcohols. Potential cytotoxic activity作者:F. J. Martínez Crespo、J. A. Palop、Y. Sainz、S. Narro、V. Senador、M. González、A. López De Ceráin、A. Monge、E. Hamilton、A. J. BarkerDOI:10.1002/jhet.5570330620日期:1996.11Several quinoxaline 1,4-di-N-oxides have been shown to be efficient and selective cytotoxins for hypoxic cells. We present now a series of 4-cyano-2-oxo-1,2,4-oxadiazolo[2,3-a]quinoxaline 5-N-oxides 2a-2k. They were prepared starting from 3-amino-2-quinoxalinecarbonitrile 1,4-di-N-oxides 1a-1k and 2-chloroethyl isocyanate in dry dioxane at 100–110°. A reaction mechanism is proposed. The treatment of几种喹喔啉1,4-二-N-氧化物已被证明是低氧细胞的有效和选择性细胞毒素。现在我们介绍一系列的4-氰基-2-氧代-1,2,4-恶二唑并[2,3- a ]喹喔啉5 - N-氧化物2a-2k。它们是在干燥的二恶烷中在100–110°下从3-氨基-2-喹喔啉腈1,a-二-N-氧化物1a-1k和2-氯乙基异氰酸酯开始制备的。提出了一种反应机理。用异氰酸苯酯处理1a得到2a。2c与硅胶反应,得到1c。化合物2a-2g将其在乙醇和2-丙醇存在下加热,得到相应的氨基甲酸酯3a-3g和4a-4g。通过加热1d和氯甲酸乙酯的混合物已经获得了化合物2d。当将氨基甲酸酯3b加热至150°时制备化合物2b。喹喔啉在有氧和低氧细胞中均作为细胞毒性剂进行了测试。最有趣的化合物是3g和4g。
-
Discovery of 1,2,3-triazole based quinoxaline-1,4-di-N-oxide derivatives as potential anti-tubercular agents作者:Singireddi Srinivasarao、Adinarayana Nandikolla、Amaroju Suresh、Augustynowicz-Kopec Ewa、Agnieszka Głogowska、Balaram Ghosh、Banoth Karan Kumar、Sankaranarayanan Murugesan、Sravani Pulya、Himanshu Aggarwal、Kondapalli Venkata Gowri Chandra SekharDOI:10.1016/j.bioorg.2020.103955日期:2020.7A series of thirty one novel 2-(((1-(substituted phenyl)-1H-1,2,3-triazol-4-yl)methoxy)carbonyl)-3-methylquinoxaline-1,4-dioxide (7a-l), 3-(((1-(substituted phenyl)-1H-1,2,3-triazol-4-yl)methoxy)carbonyl)-6-chloro-2-methylquinoxaline-1,4-dioxide (8a-l) and 2-(((1-(substituted phenyl)-1H-1,2,3-triazol-4-yl)methoxy)carbonyl)-6,7-dichloro-3-methylquinoxaline-1,4-dioxide (9a-g) analogues were synthesized一系列三十一个新颖的2-((((1-(取代苯基)-1 H -1,2,3-三唑-4-基)甲氧基)羰基)-3-甲基喹喔啉-1,4-二氧化物(7a- l),3-(((((1-(取代苯基)-1 H -1,2,3-三唑-4-基)甲氧基)羰基)-6-氯-2-甲基喹喔啉-1,4-二氧化物(8a -l)和2-(((((1-(取代的苯基)-1 H -1,2,3-三唑-4-基)甲氧基)羰基)羰基)-6,7-二氯-3-甲基喹喔啉-1,4-合成了二氧化(9a-g)类似物,使用多种分析技术进行了表征,并开发了化合物8 g和9f的单晶。评价合成的化合物的体外抗结核活性结核分枝杆菌H37Rv菌株和两种临床分离株 210和规格 192.标题化合物的最小抑菌浓度(MIC)为30.35至252.00 µM。在测试的化合物中,8e,8l,9c和9d表现出中等活性(MIC = 47.6 – 52.0 µM),8a表现出显着的抗结核活性(MIC
表征谱图
-
氢谱1HNMR
-
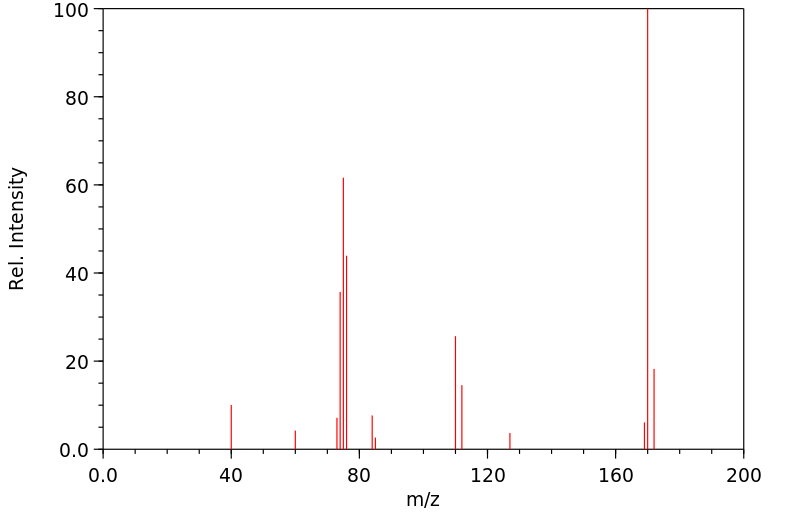
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
重氮二硝基苯酚
达罗地平
苯并芙咱-5-硼酸频那醇酯
苯并氧化呋咱-5-羧酸
苯并呋扎-5-甲腈
苯并呋喃-5-磺酰氯
苯并呋喃-5-甲酸乙酯
苯并呋喃
苯并呋咱-5-羧酸乙酯
苯并呋咱-5-羧酸
苯并呋咱-5-碳酰氯
苯并呋咱
苯并二唑-4-甲醛
苯呋咱-5-三氟硼酸钾
硝基氨基吡咯烷苯并恶嗪
哌嗪酮,6-甲基-5-硫代-,(R)-(9CI)
去甲基伊拉地平
伊拉地平内酯
伊拉地平EP杂质A
伊拉地平
乙酮,1-[5-(丁基氨基)-2-羟基苯基]-
NBD-双十六胺
N-[12-[((7-硝基-2-1,3-苯并恶二唑-4-基)氨基]十二烷酰基]-D-赤型-鞘氨醇
N-7-(4-硝基苯并-2-氧代-1,3-二氮唑)-omega-氨基己酸beta-(N-三甲基铵)乙酯
N-(7-硝基苯并-2-氧杂-1,3-二氮唑-4-基)磷脂酰乙醇胺
N-(3-氯-5-氟苯基)-4-硝基-2,1,3-苯并恶二唑-5-胺
N-(2-吗啉基乙基)-7-硝基-2,1,3-苯并恶二唑-4-胺
N,N-二甲基-7-硝基苯并呋咱-4-胺
N,N-二丁基-7-硝基-4-苯并呋咱胺
N'-[5-[[4-[5-(乙酰基-羟基氨基)戊基氨基]-4-氧代丁酰基]-羟基氨基]戊基]-N-羟基-N-[5-[(4-硝基-2,1,3-苯并恶二唑-7-基)氨基]戊基]丁二酰胺
EAM-1试剂
8-异米索前列醇
7-肼-N,N-二-4-苯并呋咱磺
7-硝基-N-[2-(2-吡啶基二硫代)乙基]-2,1,3-苯并恶二唑-4-胺
7-硝基-1-氧代-2,1,3-苯并恶二唑-1-鎓
7-甲氧基-2,1,3-苯并恶二唑-4-磺酰氯
7-氯苯并[c][1,2,5]噁二唑-4-胺
7-氯-N,N-二乙基-4-硝基-2,1,3-苯并恶二唑-5-胺
7-氯-4-硝基-5-哌啶基-2,1,3-苯并噁二唑
7-氯-4-硝基-2,1,3-苯并噁二唑1-氧化
7-氯-2,1,3-苯并噁二唑-4-磺酸
7-氟苯呋咱-4-磺酰胺
7-氟苯呋咱-4-硫氨
7-氟-2,1,3-苯并恶二唑-4-磺酰氯
7-哌啶-1-基-2,1,3-苯并恶二唑-4-胺
7-吗啉-4-基苯并[1,2,5]恶二唑-4-基胺
6-溴苯并[c][1,2,5]噁二唑1-氧化物
6-氟-2,1,3-苯并恶二唑-5-胺
6-[[7-(N,N-二甲氨基磺酰)-2,1,3-苯并恶二唑-4-基]氨基]己酸琥珀酰亚胺酯
6-[(7-硝基-2,1,3-苯并恶二唑-4-基)氨基]己酸