二丙基三硫醚 | 6028-61-1
中文名称
二丙基三硫醚
中文别名
二丙基(化)三硫
英文名称
dipropyl trisulfide
英文别名
di-n-propyl trisulfide;1,3-di(n-propyl)trisulfane;1-PROPYLTRITHIOPROPANE;1-(propyltrisulfanyl)propane
CAS
6028-61-1
化学式
C6H14S3
mdl
MFCD00040074
分子量
182.375
InChiKey
GAZXPZNJTZIGBO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:69-72 °C(Press: 1.6 Torr)
-
密度:1.076±0.06 g/cm3(Predicted)
-
LogP:5.06
-
物理描述:colourless liquid with powerful, diffusive garlic-like odour
-
溶解度:almost insoluble in water; soluble in alcohol and oils
-
折光率:1.542-1.590
-
保留指数:1313;1312;1294;1292.9;1314;1313;1302;1314;1309;1302;228.3
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:9
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:75.9
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
危险类别码:R22
-
WGK Germany:3
-
储存条件:应存放在室温、干燥且密封的环境中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Dipropyl trisulfide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Dipropyl trisulfide
CAS number: 6028-61-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H14S3
Molecular weight: 182.4
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Dipropyl trisulfide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Dipropyl trisulfide
CAS number: 6028-61-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H14S3
Molecular weight: 182.4
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:参考文献:名称:双(三苯基锡烷基)硫属元素化物对有机三硫化物的选择性单脱硫摘要:有机三硫化物在用双(三苯基锡烷基)硫属元素化物处理时,容易进行选择性的单脱硫。建议进行分子间的过程。DOI:10.1016/s0040-4039(00)77450-8
-
作为产物:描述:溴丙烷 在 sodium thiosulfate pentahydrate 、 sodiumsulfide nonahydrate 作用下, 以0.66%的产率得到二丙基三硫醚参考文献:名称:二烯丙基三硫化物 (DATS) 在还原 HAuCl4 以生产金纳米粒子中的选择性:详细研究摘要:球根大蒜(Allium sativum) 具有强烈的味道和刺鼻的气味,广泛用于烹饪制剂和民间药物。使用这种成分合成的银和金纳米粒子 (NPs) 也显示出药用和治疗效力。大蒜含有有机硫化合物,如二烯丙基硫化物 (DAS)、二烯丙基二硫化物 (DADS) 和二烯丙基三硫化物 (DATS)。这些化合物作为抗癌药物具有至关重要的意义。这里报道的是一系列具有不同取代基的 DATS 的合成,并且可能作为封端剂和还原剂来合成金纳米粒子 (TS-GNP)。在此过程中,发现在选定的 DAT 中,由于结构原因,只有 1,3-二(丁-1-烯)三硫烷可以达到目的。 图形摘要 据报道,合成了一系列具有药用意义的对称有机三硫化物作为二烯丙基三硫化物 (DATS) 的结构类似物,以尝试构建有机硫化合物诱导的金纳米粒子 (GNP)。该批次中只有 1,3-Di(but-1-ene)trisulfane 能够还原氯金酸以合成受保护的DOI:10.1007/s12039-021-01967-6
文献信息
-
Trisulfides over disulfides: highly selective synthetic strategies, anti-proliferative activities and sustained H<sub>2</sub>S release profiles作者:Debojit Bhattacherjee、Abu Sufian、Sulendar K. Mahato、Samiyara Begum、Kaustav Banerjee、Sharmistha De、Hemant Kumar Srivastava、Krishna P. BhabakDOI:10.1039/c9cc05562b日期:——Temperature- and solvent-induced selective synthesis of trisulfides and disulfides is demonstrated. A remarkable selectivity was achieved using Na2S as a sulfur-transfer agent under mild, greener, catalyst-free and additive-free conditions. This study reveals trisulfides as a better model than disulfides in general for a sustained release of H2S and potent anti-cancer activities.
-
The Reaction of Sulfur Dioxide with Thiols Catalyzed by Boron Trifluoride Etherate. Evidence for a Possible Intervention of Dithiosulfite as a Reaction Intermediate作者:Fuminori AkiyamaDOI:10.1246/bcsj.56.2657日期:1983.9The reaction of sulfur dioxide (SO2) with 1-propanethiol (NPT), 2-propanethiol (IPT), or 2-methyl-2-propanethiol (TBT) catalyzed by boron trifluoride ethrate (BF3OEt2) was investigated. The ratios of dialkyl trisulfide to dialkyl disulfide obtained (RSSSR/RSSR) at an early stage of the reaction were larger than 1 for the reaction of TBT and less than 1 for the reaction of NPT or IPT. The reaction of
-
Organic sulfur chemistry. 42. Sulfur-sulfur bond cleavage processes. Selective desulfurization of trisulfides作者:David N. Harpp、Roger A. SmithDOI:10.1021/ja00386a034日期:1982.11these transition states to alter the kinetically important step. For desulfurization without inversion at an ..cap alpha..-carbon, triphenylphosphine is quite effective as it removes almost exclusively the central sulfur atom. However, for less reactive trisulfides, a tris(dialkylamino)phosphine would be required for a rapid desulfurization. In this case, desulfurization in benzene or ether provides详细研究了叔磷化合物脱硫脱硫的选择性。提出了一种机制合理化来解释作为底物结构和溶剂极性函数的中央/末端硫挤出变化。 从这项研究得出的结论是,膦与三硫化物的相互作用是一个复杂的过程,其中两者的过渡态离子脱硫中的反应步骤具有相似的自由能,膦类型和反应溶剂的变化可能会影响这些过渡态,从而改变动力学上重要的步骤。对于没有在..cap alpha..-碳上反转的脱硫,三苯基膦非常有效,因为它几乎完全去除了中心硫原子。然而,对于活性较低的三硫化物,快速脱硫需要三(二烷基氨基)膦。在这种情况下,在苯或乙醚中脱硫提供了一种二硫化物,在一个 ..cap α..-碳(通过末端脱硫)处主要发生反转,而在乙腈中,二硫化物主要保留了两个 ..cap α.. 的立体化学。 -碳(通过中心硫去除)获得。
-
New ways of synthesis of 1,2-dithiole-3-thione作者:N. A. Korchevin、N. V. Russavskaya、G. A. Yakimova、E. N. DeryaginaDOI:10.1007/s11176-005-0095-3日期:2004.11Two new synthetic approaches to 1,2-dithiole-3-thione are proposed. The title compound is formed by thermolysis of dipropyl polysulfides (n-Pr)2Sx (x = 3–4) and thermal decomposition of polysulfide dendrimers under reduced pressure. The latter reaction may be recommended for utilization of organochlorine waste products in the manufacture of epichlorohydrin, which are used for the synthesis of dendrimers.
-
Key Odor-Active Compounds in Raw Green and Red <i>Toona sinensis</i> (A. Juss.) Roem. and Their Changes during Blanching作者:Xiaoting Zhai、Michael GranvoglDOI:10.1021/acs.jafc.0c02012日期:2020.7.8(E,Z)- and (Z,Z)-di-1-propenyl trisulfide, 2-methoxyphenol, and 4-ethylphenol were first identified as key odorants of T. sinensis. Clear differences between green and red T. sinensis in aroma profiles, flavor dilution factors, quantitative data, and odor activity values verified that (E,E)-, (E,Z)-, and (Z,Z)-di-1-propenyl disulfide, (E,E)-, (E,Z)- and (Z,Z)-di-1-propenyl trisulfide, cis- and trans-2-mercapto-3芳香提取物稀释分析和顶空香气稀释分析中的应用揭示生绿51种气味剂香椿和54种气味物质在生红香椿在8-4096风味稀释因子范围。(E,E)-2,4-癸二醛,壬醛,2,3,5-三甲基吡嗪,(E,Z)-和(Z,Z)-二-1-丙烯基三硫化物,2-甲氧基苯酚和4-乙基苯酚被首次确定为中华T的关键气味。绿色和红色中华丁香在香气特征,风味稀释因子,定量数据和气味活性值之间的明显差异证实了(E,(E)-,(E,Z)-和(Z,Z)-di-1-丙烯基二硫化物,(E,E)-,(E,Z)-和(Z,Z)-di-1-丙烯基三硫化物,顺式和反式-2-巯基3,4-二甲基-2,3-二氢噻吩和二甲基硫醚引起每个品种的明显硫磺味。此外,己醛,(E)-2-己烯醛,(E)-2-己烯-1-醇和(E,Z)-2,6-壬二烯醛导致绿色丁香中产生绿色气味。2-甲氧基苯酚和4-乙基苯酚有助于红色中华T的强烈酚类香气。变白中华绒螯蟹的定量实验和
表征谱图
-
氢谱1HNMR
-
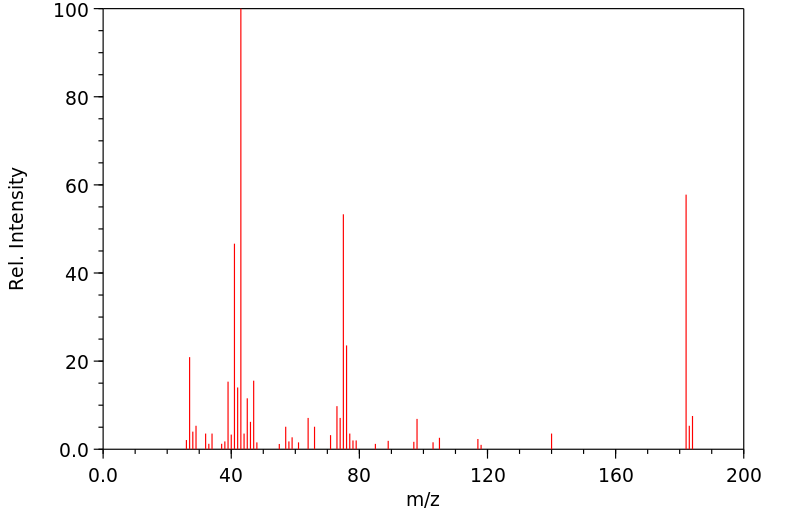
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香菇素
甲基烯丙基三硫醚
甲基乙基三硫醚
甲基丙基三硫醚
甲基(1-甲基乙基)三硫醚
大蒜粉
双十二烷基三硫醚
二甲基三硫
二甲基-d6三硫醚
二异丙基三硫醚
二叔十二烷基三硫化物
二乙基三硫醚
二丙基三硫醚
二-叔-丁基三硫化物
二(异丙氧基硫代羰基)三硫醚
二(二甲硫基氨基甲酰)三硫醚
二(乙氧基硫代羰基)三硫醚
二(三氯甲基)三硫醚
二(3-羟基丙基)三硫醚
二(2-羟基乙基)三硫醚
二(2-氯乙基)三硫醚
乙基丙基三硫醚
丙基烯丙基三硫醚
三氟-(三氟甲基硫基二硫基)甲烷
N(1),N(3)-二甲基-1,3-三硫烷二硫代甲酰胺
6-肼基-N,N-二(丙-2-烯-1-基)哒嗪-3-胺二盐酸
3,3'-三硫代二丙酰胺
1,1'-三硫代二[N,N-二丁基-硫代甲酰胺]
2-<(1-methyl-2-oxopropyl)trithio>-3-pentanone
3-<(1-methyl-2-oxopropyl)trithio>-2-pentanone
3-<(1-methyl-2-oxobutyl)trithio>-2-pentanone
diethyl 4,4'-trithiobis(butanesulfinate)
7-methyl-4,5,6,9,10-pentathiatrideca-1,12-diene
8-methyl-4,5,6,9,10-pentathiatrideca-1,12-diene
bis(1-ethyl-2-oxopropyl) trisulfide
1,4,5,6-Oxatrithiocane
2-(2,3,3,4,5,5-Hexamethylhexan-2-yltrisulfanyl)-2,3,3,4,5,5-hexamethylhexane
Di-tert-nonyl polysulfide
3,3'-(Ethane-1,2-diyl)bis(N,N-dimethyltrisulfane-1-carbothioamide)
O-Ethyl tert-butyltrisulfane-1-carbothioate
O-Ethyl butyltrisulfane-1-carbothioate
N(1),N(3)-Dibutyl-1,3-trisulfanedicarbothioamide
1,3-Trisulfanedicarbothioamide, N,N'-dicyclohexyl-
1,1'-Trithiobis(N,N-dicyclohexylmethanethioamide
di-tert-pentyl trisulfide
Dipentylxanthogentrisulfid
4,4,7,7-tetramethyl-1,2,3,5,6-pentathiepane
1-Butanesulfinic acid, 4,4'-trithiobis-, disodium salt
1,2,3,5,6-Pentathiepane, 4,7-dimethyl-
Methyl tert-butyltrisulfane-1-carboxylate