4,4-bis(hydroxymethyl)-2-methyl-2-oxazoline | 4271-18-5
中文名称
——
中文别名
——
英文名称
4,4-bis(hydroxymethyl)-2-methyl-2-oxazoline
英文别名
2-methyl-4,4-dimethylol-4,5-dihydrooxazole;2-Methyl-4,4-bis(hydroxymethyl)oxazolin;4,4-bis(hydroxymethyl)-2-methyl-1,3-oxazoline;(2-methyl-oxazole-4,4-diyl)-bis-methanol;4,4-bis-hydroxymethyl-2-methyl-4,5-dihydro-oxazole;4,4-Bis-hydroxymethyl-2-methyl-4,5-dihydro-oxazol;2-methyl-4,4-dihydroxymethyloxazoline;2-methyl-4,4(5H)-oxazoledimethanol;2-Methyl-4,4-dimethylol oxazoline;[4-(hydroxymethyl)-2-methyl-5H-1,3-oxazol-4-yl]methanol
CAS
4271-18-5
化学式
C6H11NO3
mdl
——
分子量
145.158
InChiKey
RUILRLCHVAUQDK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:95-97 °C(Solv: chloroform (67-66-3); ethyl ether (60-29-7); ethyl acetate (141-78-6))
-
沸点:150-159 °C(Press: 5 Torr)
-
密度:1.31±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-1.7
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:62
-
氢给体数:2
-
氢受体数:4
反应信息
-
作为反应物:描述:4,4-bis(hydroxymethyl)-2-methyl-2-oxazoline 在 sulfur 作用下, 以 苯 为溶剂, 20.0~100.0 ℃ 、50.66 kPa 条件下, 反应 4.0h, 生成参考文献:名称:Predvoditelev, D. A.; Savin, G. A.; Nifant'ev, E. E, Russian Journal of Organic Chemistry, 1995, vol. 31, # 4, p. 461 - 464摘要:DOI:
-
作为产物:描述:三(羟甲基)氨基甲烷醋酸盐 在 xylene 作用下, 生成 4,4-bis(hydroxymethyl)-2-methyl-2-oxazoline参考文献:名称:Nys; Libeer, Bulletin des Societes Chimiques Belges, 1956, vol. 65, p. 377,383摘要:DOI:
文献信息
-
PHOSPHATE DERIVATIVES AND USE THEREOF申请人:ZHU Qing公开号:US20210030773A1公开(公告)日:2021-02-04The present invention discloses a compound with the following formula (I), or a tautomer, mesomer, racemate, enantiomer, and diastereoisomer thereof, or a mixture form thereof, or a pharmaceutically acceptable salt thereof, or a prodrug molecule thereof, wherein D is selected from: The invention further discloses the use of the compound in the preparation of drugs for preventing and/or treating cancers, and the use of the compound in the preparation of drugs for inhibiting cancer metastasis. The compound of the present invention can effectively inhibit the proliferation and metastasis of cancer cells by adjusting the acidity of a tumor microenvironment to achieve a better effect in clinical cancer treatment, and has broad application prospects.本发明公开了具有以下式(I)的化合物,或其互变异构体、共振异构体、外消旋体、对映体和非对映异构体,或其混合形式,或其药学上可接受的盐,或其前药分子,其中D从以下中选择: 该发明还公开了该化合物在制备用于预防和/或治疗癌症的药物中的用途,以及该化合物在制备用于抑制癌症转移的药物中的用途。本发明的化合物可以通过调节肿瘤微环境的酸度来有效抑制癌细胞的增殖和转移,从而在临床癌症治疗中取得更好的效果,并具有广阔的应用前景。
-
Synthesis and stereochemistry of new 1,3-thiazolidine systems based on 2-amino-2-(mercaptomethyl)propane-1,3-diol: 4,4-bis(hydroxymethyl)-1,3-thiazolidines and c-5-hydroxymethyl-3-oxa-7-thia-r-1-azabicyclo[3.3.0]octanes作者:Cristina Morar、Carmen Sacalis、Pedro Lameiras、Albert Soran、Hassan Khartabil、Cyril Antheaume、Ioan Bratu、Oana Moldovan、Mircea DarabantuDOI:10.1016/j.tet.2013.09.070日期:2013.112-amino-2-(mercaptomethyl)propane-1,3-diol [‘2-(hydroxymethyl)cysteinol’] with aryl(di)aldehydes is reported. The resulting new class of 2-aryl-4,4-bis(hydroxymethyl)-1,3-thiazolidines is investigated by NMR and IR spectroscopy in tandem with DFT calculations, permitting structural assignments that are discussed in terms of conformational analysis, anomeric effects and ring-chain tautomerism. These acquired报道的2-氨基-2-(巯基甲基)丙烷-1,3-二醇[“2-(羟甲基)cysteinol”]的芳基的(二)醛thiaminalisation。将得到的类新的2-芳基-4,4-双(羟甲基)-1,3-噻唑烷通过NMR和IR光谱与DFT计算串联调查,从而允许了在构象分析,端基异构效应来讨论结构分配和环链互变异构现象。这些获取的数据随后被利用。用甲醛处理后,随后的(双)区域选择性和1,3-噻唑烷构建块,得到第一非对称串联的噻唑烷 - 恶唑烷稠合的系统在C-5位置单独使用官能化的非对映选择性oxaminalisation。一个意想不到的重排,它由Ar的分搬迁从1,3-噻唑烷配体与1,3-恶唑烷环,被观察为在氩环的取代有重大影响。标题双环体系,其公开的均聚物和/或异向非键合相互作用的第一单晶X射线分析,也提出。
-
胺基丙二醇类衍生物、其制备方法和其药物组 合物与用途
-
Synthesis and Conformational Analysis of the First 3-Oxa-7-thia-1- r-azabicyclo[3.3.0]-c-5-octane Single Functionalised at the C-5 Position作者:Andrada But、Pedro Lameiras、Ioan Silaghi-Dumitrescu、Carmen Batiu、Sophie Guillard、Yvan Ramondenc、Mircea DarabantuDOI:10.2174/157017810791130658日期:2010.6.1Starting from TRIS [2-amino-2-(hydroxymethyl)propane-1,3-diol] via its thia analogue, 2-amino-2- (mercaptomethyl)propane-1,3-diol double cyclocondensation with formaldehyde, we report a four steps synthesis of the first term in the title series of compounds, bearing the exploitable hydroxymethyl functionality at the C-5 position. Conformational analysis is discussed based on DFT calculations and 1H-DNMR.
-
SOLID PHASE CHANGE INK COMPOSITIONS COMPRISING OXAZOLINES申请人:Carlini Rina公开号:US20130032057A1公开(公告)日:2013-02-07A solid ink composition comprising at least one crystalline oxazoline compound and at least one amorphous component derived from a polyol, and a colorant and optional viscosity modifier, which are suitable for inkjet printing, including printing on coated paper substrates. In embodiments, the solid ink formulation comprises a blend of an amorphous and crystalline components which provides a solid ink with excellent robustness when forming images or printing on coated paper substrates.一种固体油墨组合物,包括至少一种结晶的噁唑啉化合物和至少一种来自多元醇的非晶组分,以及一种色料和可选的粘度调节剂,适用于喷墨打印,包括在涂层纸基底上打印。在实施例中,固体油墨配方包括非晶和结晶组分的混合物,提供具有卓越韧性的固体油墨,用于形成图像或在涂层纸基底上打印。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
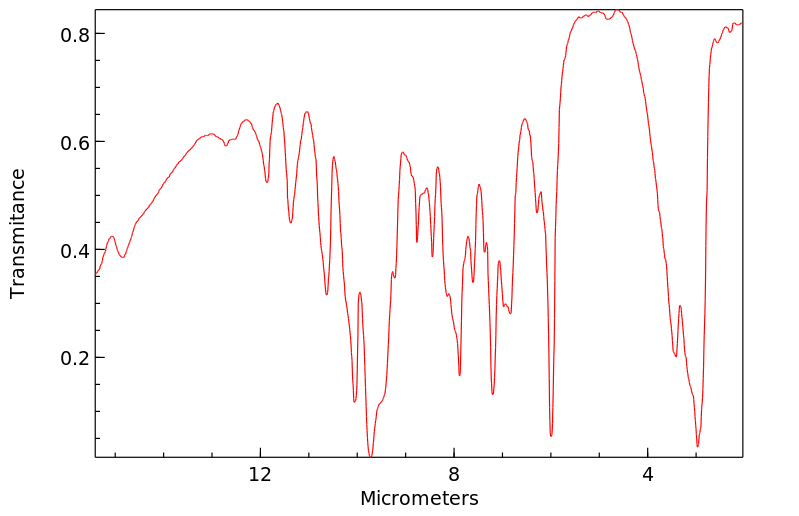
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4S,4''S)-2,2''-环亚丙基双[4-叔丁基-4,5-二氢恶唑]
香豆素-6-羧酸
顺式-3a,5,6,6a-四氢-3-(1-甲基乙基)-4H-环戊二烯并[d]异恶唑
锌离子载体IV
钐(III) 离子载体 II
苯,1-(2E)-2-丁烯-1-基-2-氟-
苯,(2,2-二氟乙烯基)-
聚二硫二噻唑烷
缩胆囊肽9
绕丹酸钠
盐(1:?)5'-尿苷酸,钠
甲酰乙内脲
甲巯咪唑
甲基羟甲基油基噁唑啉
甲基5-羟基-3,5-二甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-氰基-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基5-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-(羟基甲基)-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基4-甲基-5-氧代-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4,5-二氮杂螺[2.4]庚-5-烯-6-羧酸酯
甲基4,5-二氢-5-乙基-1H-吡唑-1-羧酸酯
甲基3-甲基-4,5-二氢-1,2-恶唑-4-羧酸酯
甲基(E)-3-[6-[1-羟基-1-(4-甲基苯基)-3-(1-吡咯烷基)丙基]-2-吡啶基]丙烯酰酸酯
甲基(5-氧代-4,5-二氢-1,2-恶唑-3-基)乙酸酯
环戊二烯并[d]咪唑-2,5(1H,3H)-二硫酮
环己羧酸,3-氨基-2-甲氧基-,甲基酯,(1S,2S,3S)-
溶剂黄93
溴化1-十六烷基-3-甲基咪唑
溴化1-十二烷基-2,3-二甲基咪唑
泰比培南酯中间体
泰比培南酯中间体
氨甲酸,[4,5-二氢-4-(碘甲基)-2-噻唑基]-,1,1-二甲基乙基酯(9CI)
氨基甲硫酸,[2-[[(2-羰基-1-咪唑烷基)硫代甲基]氨基]乙基]-,O-甲基酯
异噻唑,4,5-二氯-2,5-二氢-2-辛基-
希诺米啉
四氟硼酸二氢1,3-二(叔-丁基)-4,5--1H-咪唑正离子
四唑硝基紫
噻唑烷-2,4-二酮-2-缩氨基脲
噻唑丁炎酮
噻唑,4,5-二氢-4-(1-甲基乙基)-,(S)-
噁唑,4,5-二氢-4,4-二甲基-2-(5-甲基-2-呋喃基)-
噁唑,2-庚基-4,5-二氢-
咪唑烷基脲
吡嗪,2,3-二氢-5,6-二甲基-2-丙基-
叔-丁基3-羟基-1,4,6,7-四氢吡唑并[4,3-c]吡啶-5-羧酸酯
双吡唑啉酮