硼酸二甲酯 | 4542-61-4
中文名称
硼酸二甲酯
中文别名
——
英文名称
dimethyl boronate
英文别名
Dimethoxy-boran;dimethoxyborane
CAS
4542-61-4
化学式
C2H7BO2
mdl
——
分子量
73.8874
InChiKey
VAWRKITUPUFMHV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-130.6°C
-
沸点:25.9°C
计算性质
-
辛醇/水分配系数(LogP):-0.45
-
重原子数:5
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2920909090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Recent Developments in the Chemistry of the Boron Hydrides.摘要:DOI:10.1021/cr60098a001
-
作为产物:描述:参考文献:名称:Hydrides of Boron. III. Dimethoxyborine摘要:DOI:10.1021/ja01337a016
文献信息
-
Pyrrole derivatives申请人:——公开号:US20030181496A1公开(公告)日:2003-09-25Pyrrole derivatives represented by the following formula: 1 wherein Ring Z is an optionally substituted pyrrole ring, etc.; W 2 is —CO—, —SO 2 —, an optionally substituted C 1 -C 4 alkylene, etc.; Ar 2 is an optionally substituted aryl, etc.; W 2 and Ar 1 mean the following (1) and (2): (1) W 1 is an optionally substituted C 1 -C 4 alkylene, etc.; Ar 1 is an optionally substituted bicyclic heteroaryl having 1 to 4 nitrogen atoms as ring-forming atoms: (2) W 1 is an optionally substituted C 2 -C 5 alkylene, an optionally substituted C 2 -C 5 alkenylene, etc.; and Ar 1 is an aryl or monocyclic heteroaryl, which are substituted by carboxyl, an alkoxycarbonyl, etc. at the ortho- or meta-position thereof with respect to the binding position of W 1 , or a pharmaceutically acceptable salt thereof These compounds are useful as medicaments such as a fibrosis inhibitor for organs or tissues.以下是用中文翻译的结果: 由以下公式表示的吡咯衍生物: 其中环Z是可选择取代的吡咯环等;W 2 是—CO—,—SO 2 —,可选择取代的C 1 -C 4 烷基等;Ar 2 是可选择取代的芳基等;W 2 和Ar 1 表示如下(1)和(2): (1)W 1 是可选择取代的C 1 -C 4 烷基等;Ar 1 是具有1至4个氮原子作为环形成原子的可选择取代的双环杂芳基: (2)W 1 是可选择取代的C 2 -C 5 烷基,可选择取代的C 2 -C 5 烯基等;Ar 1 是芳基或单环杂芳基,其在与W 1 的结合位置相对应的邻位或间位处被羧基,烷氧羰基等取代, 或其药学上可接受的盐 这些化合物可用作器官或组织的纤维化抑制剂等药物。
-
Synthesis, structure, and reactivity of C-isopropyl-ortho-carborane organoboron derivatives作者:S. V. Svidlov、Ya. Z. Voloshin、N. S. Yurgina、T. V. Potapova、A. Yu. Belyy、I. V. Ananyev、Yu. N. BubnovDOI:10.1007/s11172-014-0745-x日期:2014.10A reaction of isopropyl-ortho-carborane with n-butyllithium, followed by treatment of the lithium derivative formed with boron trichloride, chlorodimethoxyborane, or chloropinacolatoborane furnished C-boryl-ortho-carboranes 1a-c. Further functionalization of 1-Cl2B-2-Pri-1,2-C2B10H10 (1a) with pentafluorophenylmagnesium bromide or pentafluorophenol led to 1-(C6F5)2B-2-Pri-1,2-C2B10H10 (2) and (1-(C6F5O)B-2-Pri-1异丙基-邻-碳硼烷与正丁基锂反应,然后用三氯化硼、氯二甲氧基硼烷或氯频那醇硼烷处理形成的锂衍生物,得到 C-硼基-邻-碳硼烷 1a-c。用五氟苯基溴化镁或五氟苯酚对 1-Cl2B-2-Pri-1,2-C2B10H10 (1a) 进行进一步官能化,生成 1-(C6F5)2B-2-Pri-1,2-C2B10H10 (2) 和 (1-( O)B-2-Pri-1,2-C2B10H10)2O (3) 分别。1-(MeO)2B-2-Pri-1,2-C2B10H10 (1b) 与 BH3 与 THF 和二甲硫醚的络合物的反应产生碳硼基硼烷加合物 4a,b。使用 1-H2B-2-Pri-1,2-C2B10H10 与二甲基硫醚 4b 的络合物作为硼氢化剂与己-1-烯和苯乙炔反应,使我们能够获得二烷基-和二(苯基烯烃)-含有 C-异丙基-邻-碳硼基硼烷,分别。C-异丙基-邻-碳硼烷基二甲氧基硼烷与三烯丙基硼烷的反应导致两个
-
Preparation of Energetic Poly(azolyl)borates as New Environmentally Benign Green-Light-Emitting Species for Pyrotechnics作者:Thomas M. Klapötke、Magdalena Rusan、Véronique SprollDOI:10.1002/zaac.201300259日期:2013.11Three different energetic poly(azolyl)borates, potassium dihydridobis(4-nitroimidazolyl)borate, sodium dihydridobis(4, 5-dinitroimidazolyl)borate, and sodium dihydridobis(3, 4,5-trinitropyrazolyl)borate, were synthesized as starting materials for metal-free environmentally benign green-light-emitting substitutes for barium salts in pyrotechnical mixtures. The compounds were analyzed by NMR and Raman
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Na: SVol.3, 3.7, page 1233 - 1245作者:DOI:——日期:——
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: B: B-Verb.13, 4.7.2, page 180 - 189作者:DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
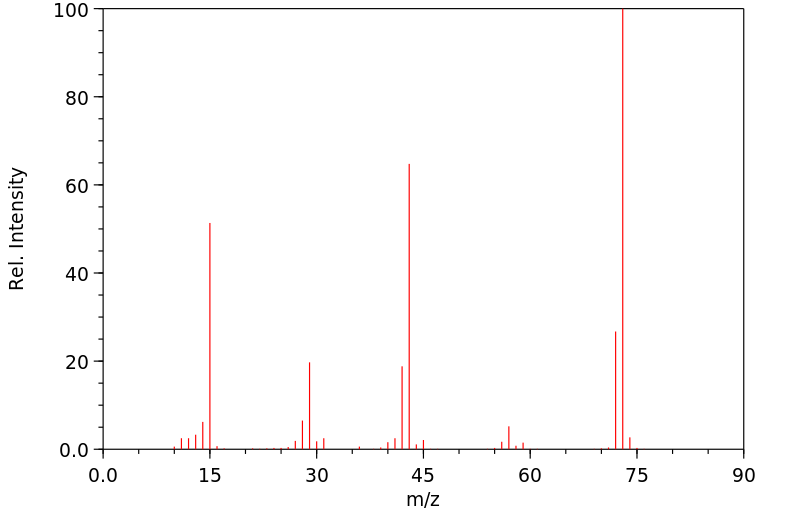
质谱MS
-
碳谱13CNMR
-
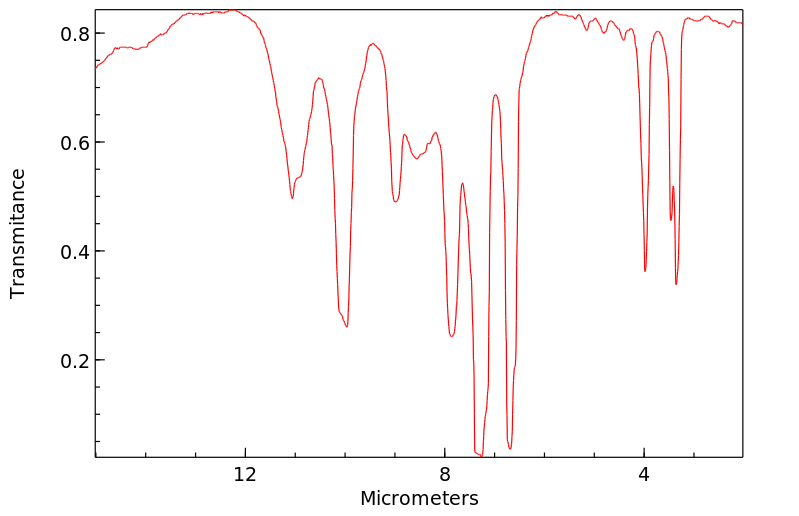
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄原酸环癸酯
高纯三甲基锑
顺式-二氯二(环丙胺)铂(II)
顺式-二氯二(乙二胺)氯化铑(1+)
顺式-二(环己基丁氨合)二氯铂(II)
顺式-二(异丙基氨合)二氯铂(II)
顺式-(2-氨基甲基-1-环戊基氨合)二氯铂(II)
顺二氯二羰基铂(II)
顺-二氯双(乙二胺)氯化铱
雷(酸)汞[含水或水加乙醇≥20]
间碳硼烷-9-硫醇
镍,加合(7:2)钪
镉二(二戊基二硫代氨基甲酸盐)
镁,溴-6-庚烯基-
manganese carbide
butyl manganese bromide
锡烷,氯二环己基-
锡四丁醇
锑,(1:1)混合物和钪
锌叔-丁氧化物
锌,溴-1-丙烯基-,(E)-
锇,加合(2:1)钪
锆酸四丁酯
锂丁酯
锂4-异丙氧基-2-甲基-丁烷-2-醇
锂1-丁醇
锂(三氟甲基)乙炔化物
锂(3-氨基丙基)酰胺
铼五羰基碘化物
铼五羰基
银(I)2-羟基乙烷-1-硫醇盐
铯三氯三羰基锇
铬三乙二胺
铬,五羰基(环己胺)-,(OC-6-22)-
铬,二(乙酰腈)二氯-
铝,加合(3:1)钪
铜-乙二胺络合物
铜(II)乙二胺
铜(I)乙炔化物
铍,环戊-1,3-二烯,溴化
铊N,N-二正丁胺
铊,甲氧基二甲基-
铂(2+)二氯化3-甲基丁烷-1,2-二胺(1:1)
铁(3+)三(1-丁醇)
铁(2+)1,1'-(硫烷二基二-1,1-乙二基)二-2,4-环戊二烯化
铀,三甲基-
钾,[三(三甲基甲硅烷基)甲基]-
钴四异硫氰酸酯
钴,乙烷-1,2-二胺
钠辛基二硫代氨基甲酸酯