溴 | 7726-95-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
制备方法与用途
主要用途包括制取溴化物,并应用于医药、农药、染料、香料、摄影材料、灭火剂、选矿、冶金、鞣革及净水等领域。作为普通分析试剂,乙烯和重碳氢化合物的吸收剂,以及有机合成中的溴化剂。
合成制备方法 1. 空气吹出法将硫酸酸化的海水(pH值为3~3.5)与氯气一同送入溴游离塔进行氧化反应。生成的游离溴进入吹出塔,通过空气将其吹出。含溴空气进入吸收塔,用纯碱溶液吸收生成溴化钠和溴酸钠。加入硫酸酸化后(pH约为2),通过蒸馏、冷凝及分离得到成品溴。
2. 蒸汽蒸馏法将预热至65~75℃的浓原卤料液加入溴反应塔,并通入氯气和蒸汽进行逆流置换反应,氧化置换出来的溴溶解于浓原卤中。通过蒸汽加热使溴汽化并随水蒸气一同从塔上部排出。温度控制在80~90℃,溴经冷凝器冷却至20~30℃后分离、精馏、冷凝制得成品溴。
3. 二氧化硫法含溴素空气与二氧化硫反应生成溴化氢气体,用水吸收并蒸馏制得溴素。
4. 工业溴的提纯采用蒸馏法去除有机杂质。装置如图所示:1—圆底烧瓶;2—柱子;3—球形接头;4—冷凝器;5—接受器;6,7—吸收瓶
为除去氯杂质,可用氢溴酸处理: [ \text{Cl}_2 + 2\text{HBr} \rightarrow \text{Br}_2 + 2\text{HCl} ]
在1~1.5L玻璃瓶中加入275mL溴,分批加入48%氢溴酸(每次25mL),观察混合物直至均匀。停止加酸后,加入200~250mL水(每次50mL)使液体分离,下层为纯溴(225g)。上层液体蒸馏可得600g溴,最后通过蒸馏回收氢溴酸。
5. 氢溴酸处理法在搅拌下将48%的氢溴酸少量分次加入工业溴中,直至混合物均匀。再依次加入蒸馏水使液体分离,下层为纯溴(225g),上层液体蒸馏收集56~61℃下蒸出的溴。与下层合并即得试剂溴。
用途简介主要用于制造无机和有机溴化合物,并应用于阻燃剂、汽油和航空燃料抗震剂的生产。在医药领域用于生成抗菌素如溴化钠、溴化钾及氯霉素、金霉素等,镇静剂如溴化樟脑。农业上用于杀虫剂、熏蒸剂及植物生长激素的生产。染料工业中使用于溴靛蓝等含溴染料制造。感光工业则用于溴化银,是制作照片、电影胶片的主要原料。石油工业中用于二溴乙烷、2-溴丙烷等产品的制造。此外,在选矿、冶金、食品、鞣革及化学分析和水处理方面也有广泛应用。
用途上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Langlois, Justus Liebigs Annalen der Chemie, 1861, vol. 1, p. 384摘要:DOI:
-
作为产物:参考文献:名称:Dancer, W., Journal of the Chemical Society, 1862, vol. 15, p. 479摘要:DOI:
-
作为试剂:描述:(3R,5R,8R,9R,10S,13S,14S,17S)-17-((R)-1-hydroxyethyl)-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-ol 在 氢溴酸 、 溴 、 potassium carbonate 、 pyridinium chlorochromate 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 为溶剂, 反应 15.0h, 生成 1-(3-fluorophenyl)-3-(2-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl)-1,3-dihydro-2H-imidazole-2-one参考文献:名称:在 C-21 处具有新型药效团的神经活性类固醇的合成和评估可以确定具有有利 PK 谱和更高 EC50 和 Emax 的化合物作为 GABAA 受体的 PAM摘要:Zuranolone (SAGE-217) 是一种神经活性类固醇 (γ-氨基丁酸)A (GABAA) 受体阳性变构调节剂 (PAM),是 FDA 于 2023 年批准的第一个口服药物,用于治疗产后抑郁症 (PPD) 患者。SAGE-217 有一个“黑框”警告,会损害驾驶或从事其他潜在危险活动的能力。此外,SAGE-217 可引起嗜睡和意识模糊等 CNS 抑制作用、自杀念头和行为以及胚胎-胎儿毒性。基于 SAGE-217 的构效关系 (SAR),设计并合成了 28 种在 C-21 调节的 SAGE-217 衍生物下具有新型药效团的神经活性类固醇。通过突触 α1β2γ2 GABAA 受体和突触外 α4β3δ GABAA 受体细胞测定评价生物活性。最佳化合物 S28 在突触外 GABAA 受体上表现出比 SAGE-217 更有效的效力和相似的疗效。与上述不同,化合物 S28 对突触 GABAA 受体的效力与DOI:10.1016/j.ejmech.2024.116602
文献信息
-
A European Perspective on Depression in the Community: The DEPRES Study作者:Saena Arbabzadeh-Bouchez、Andre Tylee、Jean-Pierre LépineDOI:10.1017/s1092852900017430日期:2002.2
ABSTRACT Depression is one of the most prevalent disorders in the general population, causing personal and social disability and impairment. Major studies assessing the diagnosis and management of depression have shown that it is often underdiagnosed and undertreated. A pan-European study aimed at assessing the extent and consequences of depression in six different countries is reported in this article. Different types of depressive profiles are analyzed and their respective management has been compared. The importance of improving diagnosis and treatment of depression is underlined. Appropriate management of depression depends on the recognition of depressive symptoms by patients, their possibility of seeking care, and the ability of the primary care physician to recognize the disorder and prescribe the appropriate medicines. Improvement in all of these fields is necessary.
摘要抑郁症是普通人群中最常见的疾病之一,会造成个人和社会的残疾和损伤。对抑郁症的诊断和管理进行评估的主要研究表明,抑郁症往往诊断不足、治疗不力。本文报告了一项泛欧研究,旨在评估六个不同国家抑郁症的程度和后果。研究分析了不同类型的抑郁症,并对其各自的治疗方法进行了比较。文章强调了改进抑郁症诊断和治疗的重要性。抑郁症的适当治疗取决于患者对抑郁症状的认识、他们寻求治疗的可能性以及初级保健医生识别疾病和开具适当药物的能力。所有这些领域都需要改进。 -
Detection of coronary stenoses by stress echocardiography using a previously implanted pacemaker for ventricular pacing: Preliminary report of a new method作者:Daniel Benchimol、Marc Mazanof、HÉLÈNe Benchimol、Virginie Bernard、Thierry Couffinhal、Raymond Roudaut、BÉNÉDicte Dubroca、Jean François Dartigues、Jacques Bonnet、Xaver PilloisDOI:10.1002/clc.4960231111日期:2000.11infarction. HYPOTHESIS To detect significant coronary stenosis in patients with previously implanted pacemakers, we tested a new stress echocardiography method using incremental ventricular pacing by already implanted pacemakers. METHODS We studied prospectively 25 consecutive patients who underwent stress echocardiography with increasing ventricular pacing up to either 85% of the age-predicted maximal背景技术具有起搏器的患者的数量一直在增加,并且其中许多患者将出现胸痛或暗示心绞痛的症状。使用起搏器的患者很难通过无创方法检测和评估心肌缺血和冠状动脉疾病。通常,就缺血甚至急性心肌梗塞而言,静止和运动期间的心电图(ECG)都很难分析。假设为了检测先前植入起搏器的患者的严重冠状动脉狭窄,我们测试了一种新的应力超声心动图方法,该方法采用已经植入的起搏器进行增量心室起搏。方法我们前瞻性地研究了连续25例接受压力超声心动图检查的患者,这些患者的心室起搏增加至年龄预测的最大心率或胸痛的85%。阳性试验是由新的运动功能减退或至少在两个相邻地区壁运动的先前变化加重定义的。所有患者均进行了冠状动脉造影,以确定冠状动脉狭窄的存在和严重程度。结果在25项测试中,有11项(44%)因胸痛而停止使用。中度不适感为1(4%),血压下降为1(4%),其余12位患者(48%)的测试中达到了目标起搏率。没有并发症。13名患
-
Complexes of platinum(II), platinum(IV), rhodium(III) and iridium(III) containing orthometallated triphenylphosphine作者:Martin A. Bennett、Suresh K. Bhargava、Mingzhe Ke、Anthony C. WillisDOI:10.1039/b004908p日期:——atoms. Oxidative addition of methyl iodide or iodine to [PtC6H4(PPh2)-2}2] gives initially platinum(IV) complexes [PtI(R)C6H4(PPh2)-2}2] (R = Me or I) in which the added groups are mutually trans; in the final, stable products the added groups and the phosphorus atoms are, separately, mutually cis. Oxidative addition of bromine to [PtC6H4(PPh2)-2}2] gives initially trans-[PtBr2C6H4(PPh2)-2}2] but用2-LiC 6 H 4 PPh 2处理[PTCl 2(SEt 2)2 ]或[RhCl 3(SEt 2)3 ],得到四元环螯合物芳基铂(II)或芳基铑(III)络合物[ PT C 6 H 4(P Ph 2)-2} 2 ]和[ Rh C 6 H 4(P Ph 2)-2} 3 ],而相应的[IrCl 3(SEt 2)3 ]给出了Ir-C 6 H 4(PPh 2)键断裂产生的IrCl C 6 H 4(P Ph 2)-2} 2(PPh 3)] 。[ PT C 6 H 4(P Ph 2)-2} 2 ]的化学性质主要是由PT–P键的不稳定性所决定的,这些键依次被配体在室温下,得到含有单齿Ç络合物6 ħ 4(PPH 2),即[铂C 6 H ^ 4(P博士2)-2} η 1 -C 6 ħ 4(PPH 2)-2}(L )] [L = PPH 3,P(OPH)3,P(OME)3或卜吨] NC和[PT
-
Accessing Pincer Bis(carbene) Ni(IV) Complexes from Ni(II) via Halogen and Halogen Surrogates作者:Gabriel Espinosa Martinez、Cristian Ocampo、Yun Ji Park、Alison R. FoutDOI:10.1021/jacs.5b12827日期:2016.4.6bis(diisopropylphenyl-benzimidazol-2-ylidene)phenyl); X = Cl or Br) with halogen and halogen surrogates to form ((DIPP)CCC)NiX3. These complexes represent a rare oxidation state of nickel, as well as an unprecedented reaction pathway to access these species through Br2 and halogen surrogate (benzyltrimethylammonium tribromide). The Ni(IV) complexes have been characterized by a suite of spectroscopic techniques
-
Reactivity of a Dimethylplatinum(II) Complex with the Bis(2-pyridyl)dimethylsilane Ligand: Easy Silicon–Carbon Bond Activation作者:Muhieddine Safa、Michael C. Jennings、Richard J. PuddephattDOI:10.1021/om3000136日期:2012.5.14(1; bps = bis(2-pyridyl)dimethylsilane) undergoes easy oxidative addition with bromine, iodine, methyl iodide, or methyl triflate to give [PtBr2Me2(bps)], [PtI2Me2(bps)], [PtIMe3(bps)], or [PtMe3(OH2)(bps)][OTf], respectively. The complex [PtIMe3(bps)] is slowly hydrolyzed in solution, with cleavage of the pyridyl–silicon bonds, to give [PtIMe3(py)2] and (Me2SiO)n. In contrast, oxidation of 1 with oxygen/CF3CH2OH化合物[PTMe 2(bPS)](1; bPS =双(2-吡啶基)二甲基硅烷)易与溴,碘,碘甲烷或三氟甲磺酸甲酯氧化加成,得到[PTBr 2 Me 2(bPS)],[ PTI 2 Me 2(bPS)],[PTIMe 3(bPS)]或[PTMe 3(OH 2)(bPS)] [OTf]。配合物[PTIMe 3(bPS)]在溶液中缓慢水解,并裂解吡啶基-硅键,得到[PTIMe 3(py)2 ]和(Me 2 SiO)n。相反,用氧气/ CF 3氧化1CH 2 OH,过氧化氢,或过氧化二苯甲酰/ H 2 ö用甲基-硅键的裂解发生,得到[PTME(BPS)-μ-κ 3 Ñ,Ñ,Ô -OSiMe(2-C 5 H ^ 4 N)2 PTME 3 ] [CF 3 CH 2 OB(C 6 ˚F 5)3 ],[PTME 3 κ 3 ñ,ñ,ø - (2-C 5 H ^ 4 N)2 SIMEO}]或[PTME 3
表征谱图
-
氢谱1HNMR
-
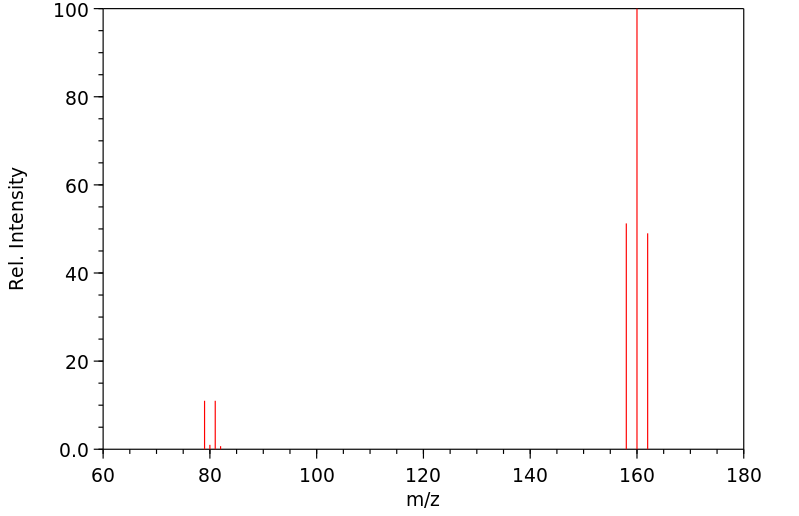
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息