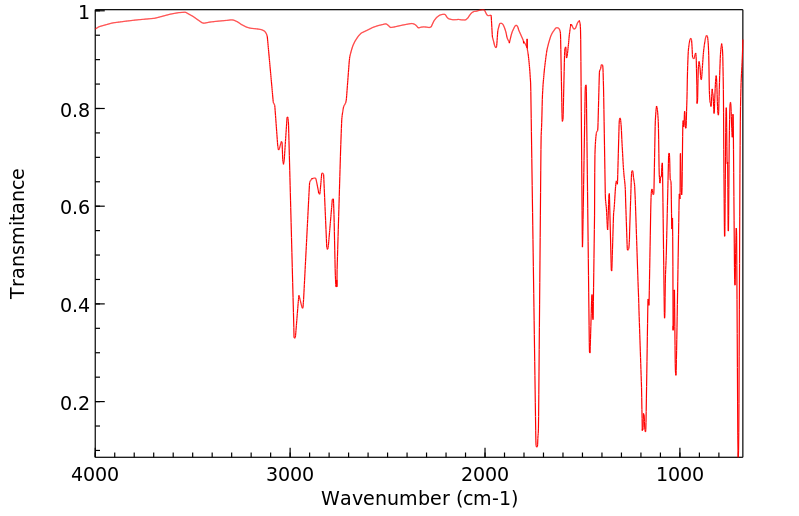
毒理性
哺乳期使用总结:哺乳期母亲口服麻醉剂可能会导致婴儿昏睡,严重的中枢神经系统抑制。新生婴儿似乎对即使是很小的剂量的丙氧芬也非常敏感,可能会特别容易导致这些效果。在哺乳期间应避免使用丙氧芬。
对哺乳婴儿的影响:在一项对12名足月哺乳新生儿进行的情况对照研究中,这些新生儿在出生后第一周出现了无法解释的呼吸暂停、心动过缓或发绀,确定母亲口服丙氧芬是可能的原因。与未发生这些症状的新生儿相比,使用阿片类药物(包括丙氧芬)进行产后镇痛的母亲所生的婴儿中,出现症状的比例更高,分别为83%和31%。受影响的新生儿母亲平均服用的剂量也更高,平均为10剂(范围4至22剂),而对照组为5剂(范围1至13剂)。
一名足月哺乳婴儿在10天大时出现轻度肌张力低下和哺乳困难;到这个时候,婴儿还没有恢复出生时的体重。7天后,母亲的乳汁减少并停止哺乳。婴儿的母亲在产后前10天被开出了每天6粒丙氧芬30毫克加扑热息痛500毫克的处方。从婴儿的头发样本中检测出了丙氧芬和去丙氧芬,且浓度高于母亲头发样本的浓度。丙氧芬可能是婴儿不良反应的原因。
法国一家药物警戒中心汇编了1985年1月至2011年6月间报告的所有哺乳婴儿的不良反应。在174份报告中,丙氧芬被报告在11名婴儿中引起不良反应,并且是在严重不良反应中最常被怀疑的药物之一。反应包括肌张力低下、呼吸暂停、呼吸窘迫、心动过缓、便秘和体重下降。
对泌乳和母乳的影响:麻醉剂可以增加血清催乳素。然而,对于已经建立泌乳的母亲来说,催乳素水平可能不会影响她的哺乳能力。
◉ Summary of Use during Lactation:Maternal use of oral narcotics during breastfeeding can cause infant drowsiness, and severe central nervous system depression. Newborn infants seem to be particularly sensitive to the effects of even small dosages of propoxyphene may be particularly prone to causing these effects. Propoxyphene should be avoided during breastfeeding.
◉ Effects in Breastfed Infants:In a case-control study of 12 breastfed term newborns with unexplained episodes of apnea, bradycardia or cyanosis during the first week of life, maternal oral propoxyphene use was determined to be the probable cause. A higher proportion of newborns with episodes, 83 compared to 31%, had mothers using opiates, including propoxyphene, for postpartum analgesia. The mean number of doses taken was also higher with mothers of affected newborns taking a mean of 10 doses (range 4 to 22) compared to 5 doses (range 1 to 13) in the control group.
A fullterm breastfed infant had mild hypotonia and nursing poorly at 10 days of age; the infant had not regained her birth weight by this time. By 7 days later, the mother had less milk and stopped nursing. The infant's mother had been prescribed 6 capsules daily of propoxyphene 30 mg plus acetaminophen 500 mg for the first 10 days postpartum. Hair samples from the infant were positive for propoxyphene and norpropoxyphene and were higher than the mother's hair sample concentrations. Propoxyphene was probably the cause of the adverse reactions in the infant.
All adverse reactions in breastfed infants reported in France between January 1985 and June 2011 were compiled by a French pharmacovigilance center. Of 174 reports, propoxyphene was reported to cause adverse reactions in 11 infants and to be one of the drugs most often suspected in serious adverse reactions. Reactions included hypotonia, apnea, respiratory distress, bradycardia, constipation and weight loss.
◉ Effects on Lactation and Breastmilk:Narcotics can increase serum prolactin. However, the prolactin level in a mother with established lactation may not affect her ability to breastfeed.
来源:Drugs and Lactation Database (LactMed)