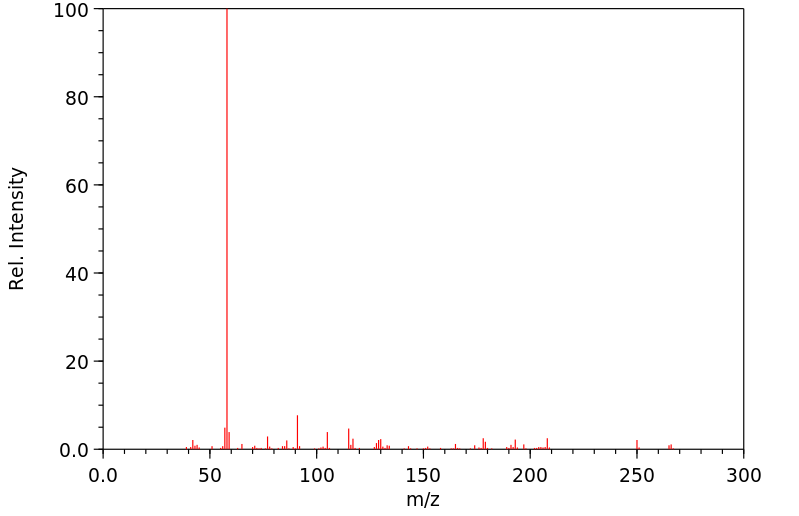
代谢
在人中,主要的代谢途径是N-去甲基化,生成去甲丙氧酚...
IN MAN, MAJOR ROUTE OF METAB IS N-DEMETHYLATION TO YIELD NORPROPOXYPHENE...
来源:Hazardous Substances Data Bank (HSDB)