(T-4)-双(1-呱啶二硫代羧酸-S,S’)-锌 | 13878-54-1
中文名称
(T-4)-双(1-呱啶二硫代羧酸-S,S’)-锌
中文别名
——
英文名称
(pentamethylenedithiocarbamate)2 Zn(II)
英文别名
Bis(piperidine-1-carbothioylsulfanyl)ZINC;zinc;piperidine-1-carbodithioate
CAS
13878-54-1
化学式
C12H20N2S4Zn
mdl
——
分子量
385.958
InChiKey
YBKBEKGVHFHCRI-UHFFFAOYSA-L
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204 °C
-
溶解度:二甲基亚砜(微溶)
计算性质
-
辛醇/水分配系数(LogP):2.61
-
重原子数:19
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:72.7
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2933399090
SDS
制备方法与用途
合成制备方法
-
五亚甲基二硫代氨基甲酸锌的制备:将六氢吡啶、二硫化碳和氢氧化钠溶液加入反应釜中,搅拌并控制反应温度在(18±3)℃之间进行缩合反应。当反应体系的pH值不再变化时,即为缩合反应终点。此时产物为N-五亚甲基二硫代氨基甲酸钠。
-
促进剂ZPD的制备:将上述制得的N-五亚甲基二硫代氨基甲酸钠加入氯化锌水溶液中,在搅拌并保持温度(18±3)℃的情况下进行复分解反应。完成反应后,产物需经水洗、过滤、干燥、粉碎和过筛处理,即可得到成品。
本品用作天然橡胶、丁苯橡胶及胶乳的超促进剂。其硫化临界温度较低,因此在混炼过程中需要特别注意防止焦烧现象的发生;当硫化温度超过121℃时,硫化平坦性会急剧变窄。与秋兰姆促进剂相比,本品具有更快的硫化速度,适用于快速加压硫化、热空气硫化以及水不溶性促进剂胶乳的硫化过程。为了获得最佳效果,通常需要配合使用氧化锌。此外,它也可用作噻唑类促进剂的第二促进剂。由于其无污染特性,硫化后的橡胶制品无臭味且性能优良,主要应用于制造胶乳制品、胶布、胶鞋及自硫化胶浆等产品。推荐用量为0.15~1份。
反应信息
-
作为反应物:描述:(T-4)-双(1-呱啶二硫代羧酸-S,S’)-锌 、 tetramethylammonium benzo[d]oxazole-2-thiolate 在 acetone 作用下, 以 丙酮 为溶剂, 生成参考文献:名称:橡胶硫化的无机化学方面。第2部分。衍生自二烷基二硫代氨基甲酸酯,2-巯基-苯并噻唑,-苯并恶唑和-苯并咪唑的阴离子混合配体锌配合物,以及[NEt 4 ] [Zn(S 2 CNMe 2)3 ],[NBu的晶体和分子结构ñ 4 ] [锌(C 7 H ^ 4 NS 2)3(OH 2)],[NBU ñ 4 ] [锌(S 2 CNME 2)2(C 7 H ^ 4 NS 2)] c ^ 2 ^ h 5OH和[NBu n 4 ] [Zn(S 2 CNMe 2)(C 7 H 4 NS 2)2 ]摘要:的配合物[锌(S 2 CNR 2)3 ] -(R = Me或Et),[锌(S 2 CNR 2)(S 2 CNR' 2)2 ] - [R =甲基,乙基,或卜Ñ ; R'= Me或Et; R 2 =(CH 2)5,R'= Me或Et],[Zn(C 7 H 4 NS 2)3(OH 2)] –(C 7 H 4 NS 2 =苯并噻唑-2-硫醇盐),[锌(S 2 CNR 2)2(C 7 H 4 NS 2)] – [R = Me,Et或Bu n;R 2 =(CH 2) 5 ],[Zn(S 2 CNR 2) 2(C 7 H 4 NOS)] – [R = Me,Et或Bu n;R 2=(CH 2) 5;C 7 H 4 NOS =苯并恶唑-2-硫醇盐],[Zn(S 2 CNR 2)(C 7 H 4 NS 2) 2 ]– [R = Me,Et,Bu n或C 6 H 11;R 2 =(CH 2) 5 ],[Zn(SDOI:10.1039/dt9800001945
-
作为产物:描述:zinc diacetate 、 sodium piperidinedithiocarbamate 在 三乙胺 作用下, 以 乙醇 、 水 为溶剂, 生成 (T-4)-双(1-呱啶二硫代羧酸-S,S’)-锌参考文献:名称:哌啶二硫代氨基甲酸酯衍生的Zn(II)和Pd(II)配合物的抗菌,计算和分子对接研究摘要:Zn(II)和Pd(II)的混合配体配合物是由哌啶二硫代氨基甲酸酯(PipDT)和胺配体{2,2'-联吡啶(Bipy),1,10-菲咯啉(Phen)和3-氨基吡啶( 3Apy)},得到的类型的配合物[M(κ 1 -PipDT)(κ 2 -Bipy)] {M II Zn,PD}(1,4),[M(κ 1 -PipDT)(κ 2 -苯)](2,5),和[M(κ 1 -PipDT)(κ 1 -3Apy)2 ](3,6)。当量的苄基异噻唑啉酸钠(Nabit)或糖精酸钠(Nasac)与顺式-[PdCl 2(PPh 3)2 ]的摩尔反应,然后添加哌啶二硫代氨基甲酸钠(NaPipDT)得到反式-[Pd(κ 1 -PipDT)(κ 1 -N位)(PPH 3)2 ](7)和反式- [钯(κ 1 -PipDT)(κ 1 -N-SAC)(PPH 3)2 ](8)。所得的配合物通过元素分析和光谱技术表征。所述PipDTDOI:10.1002/aoc.6108
文献信息
-
METHOD FOR PRODUCING ARYL, HETEROARYL, OR ALKENYL-SUBSTITUTED UNSATURATED HYDROCARBON申请人:Nishizawa Toshiaki公开号:US20120142975A1公开(公告)日:2012-06-07The present invention relates to a method for producing aryl-, heteroaryl- or alkenyl-substituted unsaturated hydrocarbons, containing: reacting aryl halides, heteroaryl halides or alkenyl halides with alkynes or alkenes in the presence of a palladium catalyst to obtain a crude product, and subsequently distillatively purifying the crude product in the presence of a compound having at least one NC═S group.
-
Synthesis, Characterization Studies and Bond Valence Sum (BVS) analysis on 2:1 Adducts Involving Zinc(II)dithiocarbamates and 4,4’-Bipyridine作者:Arumugam Manohar、Kuppukannu Ramalingam、Kottamalai KarpagavelDOI:10.13005/ojc/310269日期:2015.6.202:1 adducts involving [Zn(dtc)2]2 (dtc- = pipdtc-, H10C5NCS-2; dnpdtc-, (H3CCH2CH2)2NCS-2; dedtc-, (H5C2)2NCS-2; dmdtc-, (H3C)2NCS-2; nmedtc-, HOCH2CH2(CH)3NCS-2; deadtc-, (HOCH2CH2)2NCS-2) and 4,4' – bipyridine were prepared and characterized by elemental analyses, IR, UV-Visible, Cyclic voltammetric and thermal studies. IR spectra of the complexes show the reduction in the thioureide stretching frequency due to the increase in coordination around the zinc ion and the resultant increase in electron density. The charge transfer transitions are observed in the region 260 – 320 nm by electronic spectra. Thermal studies show a single stage weight loss. The cyclic voltammetric study on the complexes show an increase of electron density on zinc in the adducts compared to [Zn(dtc)2]2. The bond valence sum(BVS) analysis shows the values to be close to '2', which is equivalent to the formal oxidation state of zinc in the zinc complexes.2:1 加合物涉及 [Zn(dtc)2]2 (dtc- = pipdtc-, H10C5NCS-2; dnpdtc-, (H3CCH2CH2)2NCS-2; dedtc-, (H5C2)2NCS-2; dmdtc-, (H3C)制备了 2NCS-2; nmedtc-、HOCH2CH2(CH)3NCS-2; deadtc-、(HOCH2CH2)2NCS-2) 和 4,4' – 联吡啶,并通过元素分析、红外、紫外-可见、循环伏安和热分析进行表征。研究。配合物的红外光谱显示,由于锌离子周围配位的增加以及由此导致的电子密度的增加,硫脲伸缩频率降低。通过电子光谱在 260 – 320 nm 区域观察到电荷转移跃迁。热研究显示单阶段减重。对配合物的循环伏安研究表明,与 [Zn(dtc)2]2 相比,加合物中锌上的电子密度有所增加。键价和 (BVS) 分析显示值接近“2”,这相当于锌配合物中锌的形式氧化态。
-
Electron density distribution studies on ZnS4N2 chromophore in solution and solid phases: XPS and cyclic voltammetric studies on 1,10-phenanthroline and 2,2′-bipyridine adducts of bis(piperidinecarbodithioato-S,S′)zinc(II). Single crystal X-ray structure of (2,2′-bipyridine)bis-(piperidinecarbodithioato-S,S′)zinc(II)作者:S. Thirumaran、K. Ramalingam、G. Bocelli、A. CantoniDOI:10.1016/s0277-5387(98)00353-2日期:1999.2(1,10-phenanthroline)bis(piperidinecarbodithioato-S,S')zinc(II), [Zn(pipdtc)(2)(1,10-phen)] (1) and (2,2'-bipyridine)bis(piperidinecarbodithioatao-S,S')zinc(II), [Zn(pipdtc)(2)(bipy)] (2) adducts were prepared and the crystal structure of 2 is reported. The Zn-S distances in 2 are longer than those in Zn(dtc)(2) complexes. The presence of an additional neutral ligand causes an increase of the Zn-S bond lengths. S2p binding energies show a significant reduction in value compared to the parent dithiocarbmate [Zn(pipdtc)(2)](3), indicating the weakening of the Zn-S bond on adduct formation. The observed reduction in binding energy is due to the increased electron density on the metal in the adducts. The cyclic voltammetric study on the complexes also show an increase of electron density on zinc in the adducts compared to Zn(pipdtc)(2). The S-Zn-S angle in the adduct shows a reduction due to increased coordination around zinc, The thioureide C-N distance of 1.343(13) Angstrom in compound 2 is in line with the nu C-N observed at 1469 cm(-1). (C) 1999 Elsevier Science Ltd. All rights reserved.下列中文文本是所给英文内容的翻译: (1,10-邻菲罗啉)双(哌啶羰基二硫代酸-S,S')锌(II)配合物,[Zn(pipdtc)₂(1,10-phen)](1)和(2,2'-联吡啶)双(哌啶羰基二硫代酸-S,S')锌(II)配合物,[Zn(pipdtc)₂(bipy)](2)已被制备,且报道了配合物2的晶体结构。配合物2中的Zn-S键长度比Zn(dtc)₂配合物中的Zn-S键长更长。额外中性配体的存在导致Zn-S键长增加。S2p结合能的值与母体二硫代碳酸盐[Zn(pipdtc)₂](3)相比有显著降低,表明在形成加成配合物后,Zn-S键强度减弱。结合能的降低是由于加成配合物中金属上的电子密度增加所致。对配合物进行的环伏安研究还表明,与Zn(pipdtc)₂相比,加成配合物中锌上的电子密度增加。由于锌周围配位的增加,加成物中S-Zn-S键角减小。化合物2中硫脲C-N键长为1.343(13)Å,与在1469 cm⁻¹处观测到的C-N一致。 版权信息:(C) 1999 Elsevier Science Ltd. 保留所有权利。
-
Polymerizable composition, optical material comprising the composition and method for producing the material申请人:——公开号:US20040254258A1公开(公告)日:2004-12-16A cured matter obtained by polymerizing and curing a polymerizable composition comprising a compound having at least one episulfide in a molecule is reduced in an odor by adding a perfume to the polymerizable composition comprising the compound having at least one episulfide in a molecule. A cloudiness of the lens is removed by raising a purity of sulfur to 98% or more. A reduction in a chlorine content of the sulfur compound described above to 0.1% by weight or less elevates an oxidation resistance and a light fastness of the high refractive index optical material and improves a color tone thereof. An optical product having a high refractive index and a high-degree transparency is provided by subjecting the composition in advance to deaerating treatment at 0 to 100° C. for one minute to 24 hours under a reduced pressure of 0.001 to 50 torr.
-
BICYCLIC HETEROCYCLE DERIVATIVES FOR THE TREATMENT OF PULMONARY ARTERIAL HYPERTENSION申请人:Bruce Ian公开号:US20150025059A1公开(公告)日:2015-01-22Bicyclic heterocyclic derivatives of formula I useful in inhibiting PDGF receptor mediated biological activity. wherein A is and R 1 , R 1a , R 2 , R 3 , R 4 , R 5 , R 6 and X are as defined herein.
表征谱图
-
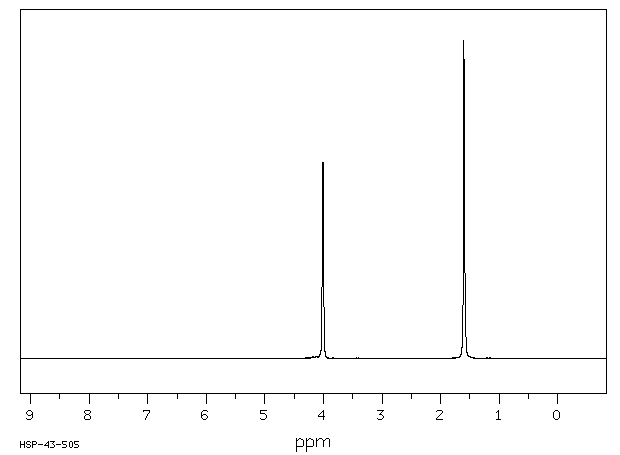
氢谱1HNMR
-
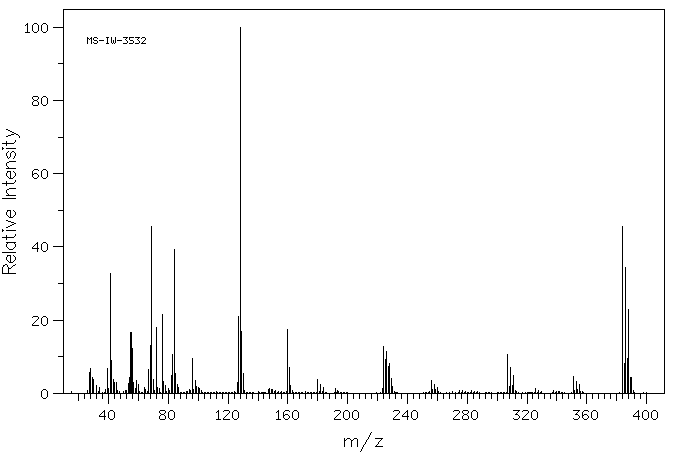
质谱MS
-
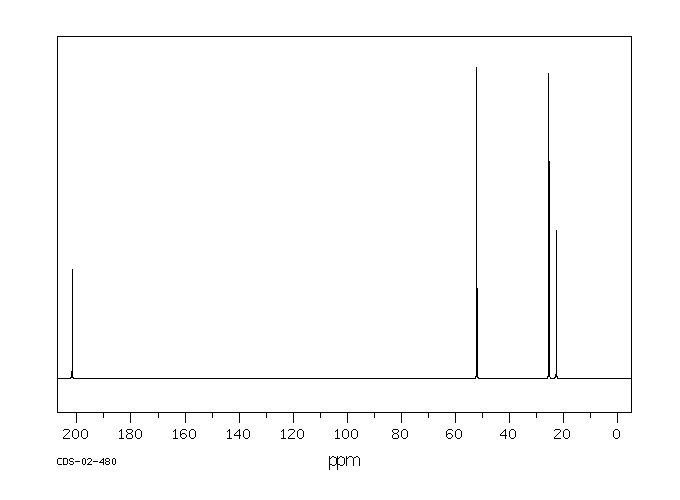
碳谱13CNMR
-
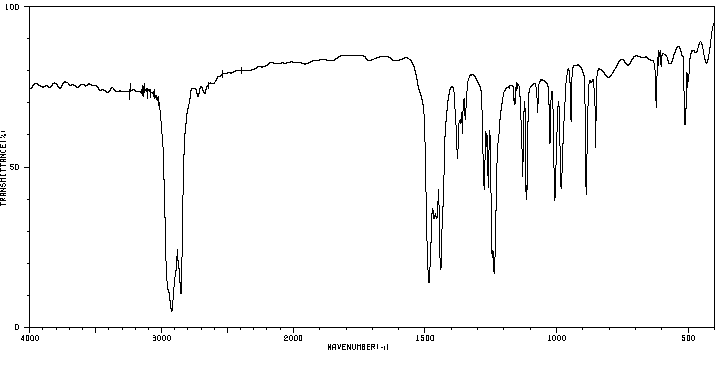
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺