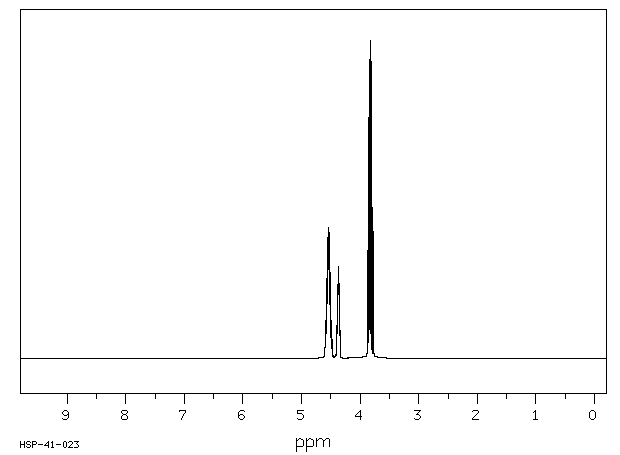
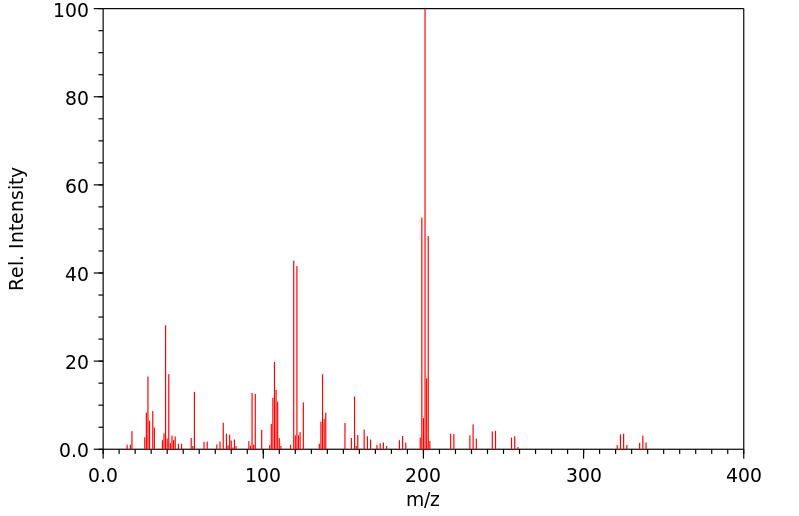
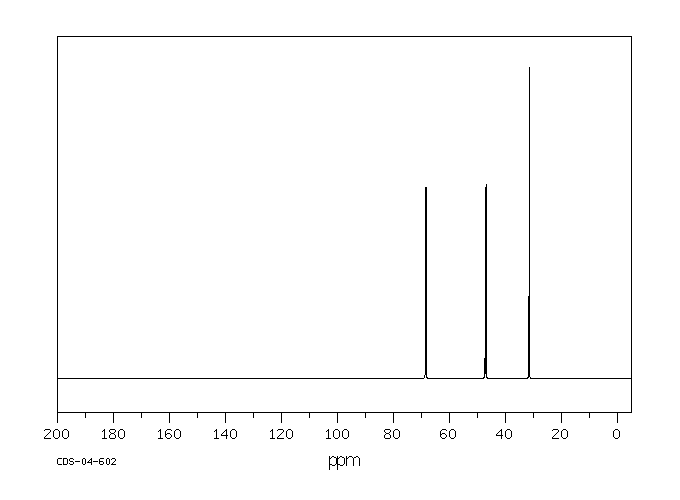
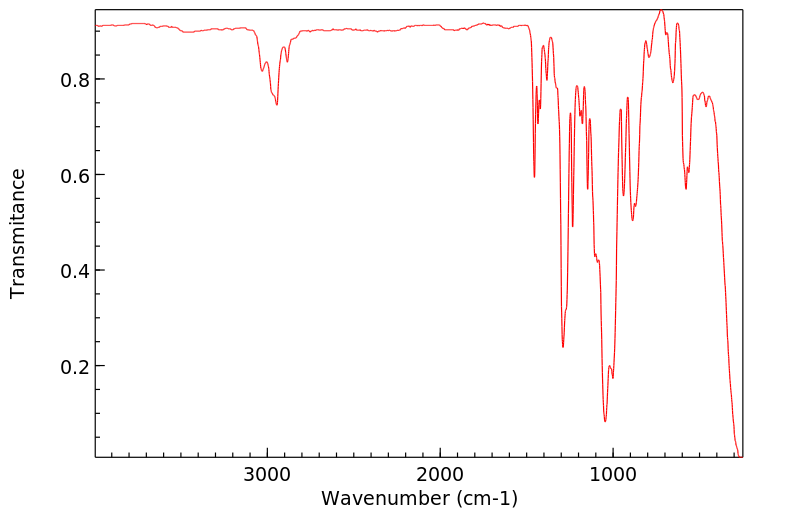
代谢
当100毫克/动物的剂量应用于雄性刘易斯大鼠剃光的皮肤上时,代谢
水解产物2,3-二
溴丙醇以自由态和结合态在尿液中可检测到数天。
来源:Hazardous Substances Data Bank (HSDB)
代谢
氧化代谢阻燃剂三-(2,3-二
溴丙基)
磷酸盐的大鼠肝微粒体产生了一种致突变代谢物。一种可能是O-脱烷基化到
2,3-二溴丙醛,这是一种已知的烷基化剂。
来源:Hazardous Substances Data Bank (HSDB)
代谢
六种代谢物,是三
溴化
双酚A通过脱烷基和脱氢
溴化反应产生的,在大鼠体内通过静脉注射和口服给药后,在尿液和胆汁中被识别,并且在体外通过大鼠肝脏中的还原型烟酰胺
腺嘌呤二核苷酸依赖性微体酶形成。主要代谢物是双(2,3-二
溴丙基)
磷酸酯,其他包括三(2,3-二
溴丙基)
磷酸酯;2,3-二
溴丙醇;2-
溴-2-
丙烯基;
2,3-二溴丙基磷酸酯;双(2-
溴-2-
丙烯基)
磷酸酯;
2,3-二溴丙基磷酸酯;以及2-
溴-2-
丙烯基
磷酸酯。
来源:Hazardous Substances Data Bank (HSDB)
代谢
强突变剂
2-溴丙烯醛是通过将致癌性阻燃剂三(2,3-二
溴丙基)
磷酸盐(tris-bp)与肝脏微粒体一起孵化形成的。用
氘替换tris-bp末端碳原子(C-3)上的氢,显著降低了突变响应和
2-溴丙烯醛的形成速率。通过质谱分析从选择性地
氘化的tris-bp类似物形成的
2-溴丙烯醛,揭示了
2-溴丙烯醛形成的主要机制是在C-3位置首先发生氧化脱卤反应,然后发生β-消除反应。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于三(2,3-二
溴丙基)
磷酸盐的人类致癌性,人类证据不足。对于三(2,3-二
溴丙基)
磷酸盐的致癌性,实验动物证据充分。总体评估:三(2,3-二
溴丙基)
磷酸盐可能对人类致癌(2A组)。在进行总体评估时,工作组考虑到三(2,3-二
溴丙基)
磷酸盐在广泛的哺乳动物体内和体外测试系统中均表现出活性。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
三(2,3-二
溴丙基)
磷酸盐根据实验动物研究中充分的致癌性证据,合理预期为人类致癌物。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:三(2,3-二
溴丙基)
磷酸盐
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:2A组:可能对人类致癌
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第20卷:(1979年)一些卤代烃
增补第7卷:致癌性的总体评估:更新国际癌症研究机构专著第1至42卷,1987年;440页;ISBN 92-832-1411-0(已绝版)
第71卷:(1999年)对一些有机
化学品、
肼和
过氧化氢的再评估(第1部分,第2部分,第3部分)
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
大约180微克/天(9微克/千克体重)的
磷酸三(2,3-二
溴丙基)通过穿着用
磷酸三(2,3-二
溴丙基)处理过的涤纶睡衣的儿童的皮肤被吸收。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Tris-BP是一种阻燃剂,通过口服和静脉注射14C标记的
Tris-BP研究了其在雄性大鼠体内的代谢和分布。
Tris-BP能够被消化道快速吸收并在体内广泛分布。无论是口服还是静脉注射,
Tris-BP来源的放射性物质的分布和排泄情况相似。给药方式对组织分布的影响主要体现在口服给药后肝脏中浓度略高,而静脉注射后肺中浓度略高。24小时内,
Tris-BP来源的放射性物质通过尿液、粪便和
二氧化碳排出的比例约占剂量的50%。给药一天后,分析组织中的
Tris-BP来源的放射性物质表明,所有组织中大部分放射性物质以各种代谢物的形式存在,而非母体化合物。大多数研究中组织的
Tris-BP来源的放射性物质的最终清除最好用单一组分指数衰减来描述,半衰期约为2.5天。肝脏和肾脏的清除速度较慢,半衰期约为3.8天。大约33%的尿液中排出的放射性物质和大约50%的胆汁中排出的放射性物质通过高效
液相色谱(HPLC)与合成标准共色谱法鉴定。通过这种方法,在尿液和胆汁中鉴定出六种代谢物和微量的母体化合物。这六种代谢物是母体化合物脱烷基和脱氢
溴反应的产物。从尿液和胆汁中分离出的
Tris-BP的代谢物也可以通过大鼠肝脏的
NADPH依赖性微粒体酶在体外形成。肝脏的可溶性酶将
Tris-BP代谢至少三种未识别的极性代谢物。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
三(2,3-二
溴丙基)
磷酸盐被从喂食100和1000毫克/千克饮食的雄性断奶大鼠的消化系统中吸收,持续28天。在28天的喂养后,在大鼠的肌肉、肝脏和脂肪中检测到与剂量相关的
溴浓度;在停止给药6周后,这些浓度降至对照
水平。当兔子每天通过皮肤应用500、1000和2000毫克/千克饮食的三(2,3-二
溴丙基)
磷酸盐时,其吸收呈剂量依赖性,通过血液中
溴化物
水平的增加可以证明。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
1972年至1976年间,大量婴儿和儿童可能通过皮肤吸收和偶尔通过口腔接触处理过的衣物摄入,接触了三(2,3-二
溴-1-丙基)
磷酸酯(TRIS)。
来源:Hazardous Substances Data Bank (HSDB)