3-(5-nitrothiophen-2-yl)propenoic acid | 17163-22-3
中文名称
——
中文别名
——
英文名称
3-(5-nitrothiophen-2-yl)propenoic acid
英文别名
3-(5-nitro-2-thienyl)acrylic acid;3-(5-nitro-thiophen-2-yl)-acrylic acid;3-<5-Nitro-thienyl-(2)>-acrylsaeure;3-(5-Nitro-2-thienyl)acrylsaeure;3-(5-nitrothiophen-2-yl)prop-2-enoic acid
CAS
17163-22-3
化学式
C7H5NO4S
mdl
——
分子量
199.187
InChiKey
IIJCRPJUZLOCIE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:251-252 °C (decomp)(Solv: ethanol (64-17-5))
-
沸点:392.2±32.0 °C(Predicted)
-
密度:1.576±0.06 g/cm3(Predicted)
-
溶解度:可溶于二氯甲烷、DMSO、甲醇
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:111
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (5-nitro-2-thienyl)methylenemalonic acid 50981-19-6 C8H5NO6S 243.197 5-硝基噻吩-2-甲醛 5-nitro-2-thenaldehyde 4521-33-9 C5H3NO3S 157.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-<5-Nitro-thienyl-<2>>-acrylsaeure-morpholid 91088-35-6 C11H12N2O4S 268.293
反应信息
-
作为反应物:参考文献:名称:Synthesis and preliminary biological evaluation of novel N-(3-aryl-1,2,4-triazol-5-yl) cinnamamide derivatives as potential antimycobacterial agents: An operational Topliss Tree approach摘要:A series of novel N-(3-aryl-1,2,4-triazol-5-yl) cinnamamide derivatives were designed on basis of structural similarity to the known FAS II inhibitors. Topliss operational method was used to optimize the potency of molecules. The minimum inhibitory concentration (MIC) of all synthesized compounds was determined against Mycobacterium tuberculosis H(37)R(v) using resazurin microtitre assay (REMA) plate method. The synthesized compounds exhibit antimycobacterial activity in the range of 5-95 mu M with a good safety profile. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2011.08.076
-
作为产物:描述:(5-nitro-2-thienyl)methylenemalonic acid 在 乙酸酐 作用下, 反应 0.25h, 生成 3-(5-nitrothiophen-2-yl)propenoic acid参考文献:名称:[EN] PROPENAMIDE THIOPHENE DERIVATIVES AS FLAVIVIRUS INHIBITORS AND THEIR USE
[FR] DÉRIVÉS DE THIOPHÈNE PROPÉNAMIDE UTILISÉS COMME INHIBITEURS DE FLAVIVIRUS ET LEUR UTILISATION摘要:本发明涉及新的黄病毒抑制剂,包括所述抑制剂的组合物和用于治疗与病毒感染有关的疾病的方法,例如由于黄病毒感染引起的疾病,包括给予所述抑制剂。公开号:WO2017102014A1
文献信息
-
Studies on the Synthesis of New Antimicrobials. II. Syntheses of 3-(5-Nitro-2-thienyl) acrylic Acid Derivatives and their Some Antibacterial Activities作者:Ryuichi Kimura、Takahiro Yabuuchi、Masakatsu HisakiDOI:10.1248/cpb.10.1232日期:——3-(5-Nitro-2-thienyl) acrylic acid was synthesized in good yield by direct nitration of 3-(2-thienyl) acrylic acid obtained from 2-thiophenecarboxaldehyde without proceeding through 5-nitro-2-thiophenecarboxaldehyde diacetate. By condensation of the acid with various amines, alcohols or phenols, its amide and ester derivatives were prepared. 2-Bromo-3-(5-nitro-2-thienyl) acrylic acid esters were also obtained by bromination of the corresponding esters. Antibacterial activity of these compounds was tested on some bacteria in vitro.
-
一类截短侧耳素芳杂环丙烯酸酯类化合物及其合成方法和应用
-
NEW FLUORESCENT CROSSLINKED AROMATIC POLYAMIDES CONTAINING THIOPHENEAND FURANE IN THEIR BACKBONE作者:C. O SÁNCHEZ、P SOBARZO、N GATICA、D MAC-LEOD-CAREYDOI:10.4067/s0717-97072015000300014日期:——New fluorescent crosslinked aromatic polyamides containing phenylen, thiophene and furane groups in the main chain were synthesized by self-condensation from 3-(5-aminothiophen-2-yl) propenoic acid, 3-(5-aminofuran-2-yl) propenoic acid and 3-(4-((5-aminothiophen-2-yl) methyleneamino) phenyl) propenoic acid using the phosphorylation method and triphenylphosphite as initiator. The amino compounds were obtained from 3-(5-nitrothiophen-2-yl) propenoic acid, 3-(5-nitrofuran-2-yl) propenoic acid and 3-(4-((5-nitrothiophen-2-yl) methyleneamino) phenyl) propenoic by selective reduction of each nitro group and was confirmed by H-1-NMR and FT-IR spectroscopy. Crosslinking of polyamides ocurr by mechanism between vinyl side groups reacts with the vinyl carbon of another chain, giving rise to interchain linear crosslinking. Depending on the structure the polymers have higher degree of crosslinking. Some vinyl groups react by thermal treatmentat 200 degrees C. Partially crosslinked polyamides with high emission fluorescence were obtained. Consequently amide bond formation, crosslinking and conjugation are the main factors that influence the fluorescence process. While polymers have several factors that affect the fluorescence, we believe that the most significant is the crosslinking of vinyl bonds. Subsequent thermal treatment of polyamides provoked crosslinking increase and fluorescence loss. The effect of the chemical structure was correlated with the thermal decomposition. Polyamides were characterized by UV-visible, FT-IR and 1H-NMR spectroscopy, inherent viscosity and thermogravimetric analysis (TGA). The synthesized polyamides exhibited potential for heat sensitive devices application since the fluorescence can be activated or quenched according to a heating process.
表征谱图
-
氢谱1HNMR
-
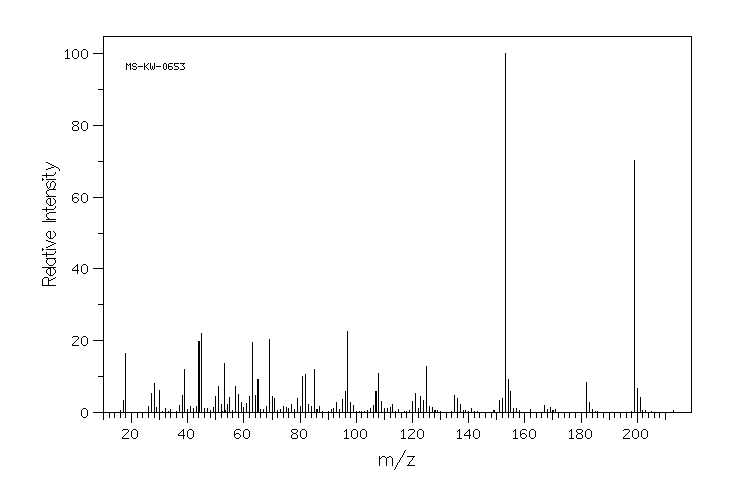
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯