diisopropyl 1-(8-dimethylamino-1-naphthyl)-1H-1,2,3-triazole-4,5-dicarboxylate
中文名称
——
中文别名
——
英文名称
diisopropyl 1-(8-dimethylamino-1-naphthyl)-1H-1,2,3-triazole-4,5-dicarboxylate
英文别名
dipropan-2-yl 1-[8-(dimethylamino)naphthalen-1-yl]triazole-4,5-dicarboxylate
CAS
——
化学式
C22H26N4O4
mdl
——
分子量
410.473
InChiKey
UOBDKJYTOWTJTN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:30
-
可旋转键数:8
-
环数:3.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:86.6
-
氢给体数:0
-
氢受体数:7
反应信息
-
作为反应物:描述:diisopropyl 1-(8-dimethylamino-1-naphthyl)-1H-1,2,3-triazole-4,5-dicarboxylate 以 甲醇 为溶剂, 反应 0.5h, 以16%的产率得到diisopropyl 9-dimethylamino-1H-benz[g]indole-2,3-dicarboxylate参考文献:名称:Synthesis and Spectroscopic Properties of 9-Substituted Benz[g]indoles摘要:Photolysis of 1,1'-(1,8-naphthylene)-di-1H-1,2,3-triazoles in methanol has given new benz[g]indoles with a triazole ring at 9-position. Similar photolysis of 1-(8-dimethylamino-1-naphthyl)-1H-1,2, 3-triazoles also gives new benz[g]indoles with a dimethylamino group at 9-position. The spectral properties of these compounds were studied in comparison with those of corresponding benz[g]indoles obtained from the similar photolysis of 1-(1-naphthyl)-1H-1,2, 3-triazoles. Since the substituent at 9-position and the pyrrole moiety exist in close proximity in peri- position of the naphthalene ring, unique properties such as the strong intramolecular hydrogen bonding and the restricted rotation of C(sp2)-N(sp3) single bond were observed in the 9-substituted benz[g]indoles.DOI:10.3987/com-98-8467
表征谱图
-
氢谱1HNMR
-
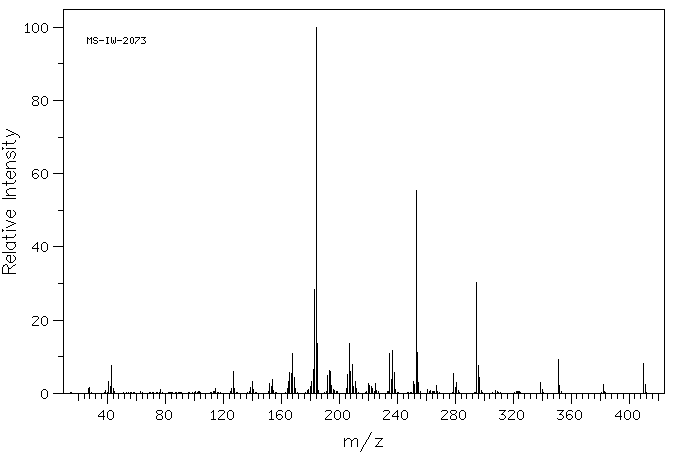
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮