methyl 5-O-trityl-α-L-arabinofuranoside | 14645-36-4
中文名称
——
中文别名
——
英文名称
methyl 5-O-trityl-α-L-arabinofuranoside
英文别名
Methyl 5-O-trityl-alpha-l-arabinofuranoside;(2R,3R,4R,5S)-2-methoxy-5-(trityloxymethyl)oxolane-3,4-diol
CAS
14645-36-4
化学式
C25H26O5
mdl
——
分子量
406.478
InChiKey
ZFZBMSNSNORRPT-CJRSTVEYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:30
-
可旋转键数:7
-
环数:4.0
-
sp3杂化的碳原子比例:0.28
-
拓扑面积:68.2
-
氢给体数:2
-
氢受体数:5
上下游信息
反应信息
-
作为反应物:描述:methyl 5-O-trityl-α-L-arabinofuranoside 在 N-碘代丁二酰亚胺 、 4 A molecular sieve 、 sodium methylate 、 silver trifluoromethanesulfonate 、 sodium hydride 、 对甲苯磺酸 作用下, 以 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 10.25h, 生成 methyl 5-O-(2,3-anhydro-β-L-lyxofuranosyl)-2,3-di-O-benzyl-α-L-arabinofuranoside参考文献:名称:糖苷键合成中的 2,3-脱水糖。含有α-和β-阿拉伯呋喃糖基键的寡糖的高度立体选择性合成摘要:由碳水化合物介导的生物学重要事件的不断发现引起了人们对寡糖合成和糖苷键形成新方法开发的极大兴趣。在本文中,我们报告了 2,3-脱水呋喃糖硫糖苷 (1, 5) 和糖基亚砜 (2, 6),其中羟基 C-2 和 C-3 被“保护”为环氧化物,糖基化醇与极高的立体声控制度。与这种全新类型的糖基化剂反应的主要或唯一产物是其中新形成的糖苷键与环氧化物部分顺式。我们进一步证明,随后环氧化物部分的亲核打开在碱性条件下进行,以高产率和良好至极好的区域选择性得到产物。主要的开环产物具有阿拉伯立体化学,因此该方法构成了合成阿拉伯呋喃糖苷的新方法。在环...DOI:10.1021/ja029302m
-
作为产物:描述:methyl 2,3,5-tri-O-benzoyl-α-L-arabinofuranoside 在 吡啶 、 sodium methylate 作用下, 以 甲醇 、 二氯甲烷 为溶剂, 反应 6.0h, 生成 methyl 5-O-trityl-α-L-arabinofuranoside参考文献:名称:糖苷键合成中的 2,3-脱水糖。含有α-和β-阿拉伯呋喃糖基键的寡糖的高度立体选择性合成摘要:由碳水化合物介导的生物学重要事件的不断发现引起了人们对寡糖合成和糖苷键形成新方法开发的极大兴趣。在本文中,我们报告了 2,3-脱水呋喃糖硫糖苷 (1, 5) 和糖基亚砜 (2, 6),其中羟基 C-2 和 C-3 被“保护”为环氧化物,糖基化醇与极高的立体声控制度。与这种全新类型的糖基化剂反应的主要或唯一产物是其中新形成的糖苷键与环氧化物部分顺式。我们进一步证明,随后环氧化物部分的亲核打开在碱性条件下进行,以高产率和良好至极好的区域选择性得到产物。主要的开环产物具有阿拉伯立体化学,因此该方法构成了合成阿拉伯呋喃糖苷的新方法。在环...DOI:10.1021/ja029302m
文献信息
-
N.m.r. spectral study of α- and β-l-arabinofuranosides作者:Kenji Mizutani、Ryoji Kasai、Midori Nakamura、Osamu Tanaka、Hiromichi MatsuuraDOI:10.1016/0008-6215(89)84018-2日期:1989.1-arabinofuranosides of various aliphatic alcohols were synthesized and then investigated by n.m.r. spectroscopy. The 13 C-n.m.r. glycosylation shift of these l -arabinofuranosides is very similar to that of l -arabinopyranosides and other glycopyranosides reported previously. The 3 J H-1,H-2 value of these α- l -arabinofuranosides is significantly different from that of the β anomers and can be used
-
Regioselective and stereocontrolled syntheses of protected L-glycosides from L-arabinofuranosides作者:Grigorii G. SivetsDOI:10.1016/j.carres.2019.107901日期:2020.2method for selective 3(2)-O-acylation of 5-O-silyl (trityl) l-arabinofuranosides was investigated based upon generation of organoboron compounds using l-Selectride and subsequent reaction of salt carbohydrate species with pivaloyl or 4-chlorobenzoyl chloride as the electrophile. Syntheses of methyl 2,3-anhydro-l-furanosides were accomplished from selectively protected methyl l-arabinofuranosides. 2(3)-D通过区域选择性碱催化甲基α-和β-1-阿拉伯呋喃糖苷与酰氯的酰化反应,描述了新的单-和二-O-保护的1-阿拉伯呋喃糖苷衍生物的合成。基于使用1-Selectride生成有机硼化合物并随后将盐类碳水化合物与新戊酰基或4-氯苯甲酰基反应的基础上,研究了一种新方法,用于5-O-甲硅烷基(三苯甲基)1-阿拉伯呋喃糖苷的选择性3(2)-O-酰化反应氯化物作为亲电试剂。甲基2,3-脱水-1-呋喃糖苷的合成是由选择性保护的甲基1-阿拉伯呋喃糖苷完成的。包括2-脱氧-1-核糖呋喃糖苷的2(3)-脱氧-1-戊呋喃糖苷及其5-O-封闭的衍生物是通过用1-Selectride将2,3-脱水-1-呋喃糖苷立体选择性还原而制备的。
-
Gram-Scale Synthesis of the C14–C23 Fragment of Eribulin作者:Qiuhan Yu、Yueer Zhou、Xinai Gao、Shuheng Pan、Feng Lin、Wei LiDOI:10.1021/acs.oprd.2c00370日期:2023.2.17synthetic efforts toward eribulin, it is still a challenge to prepare eribulin in a large scale due to its complex molecular architecture. Herein, we report a novel and efficient route to the C14–C23 fragment of eribulin from cheap l-arabinose.
-
一种抗癌药物中间体及其制备方法申请人:中国药科大学公开号:CN116063257A公开(公告)日:2023-05-05本发明公开了一种抗癌药物中间体及其制备方法。该方法从阿拉伯糖出发,通过14步反应得到目标化合物。本发明方法操作简单,收率高,成本低,适用于工业化生产。
-
Stereoselective synthesis of a novel Galf-disaccharide mimic: β-d-galactofuranosyl-(1-5)-β-d-galactofuranosyl motif of mycobacterial cell walls作者:Chunyan Liu、Hong Kang、Richard H. Wightman、Shende JiangDOI:10.1016/j.tetlet.2012.12.018日期:2013.3A Galf-disaccharide mimic, an analogue of the acceptor substrates of mycobacterial Galf-transferases, was obtained by nucleophilic addition of a L-arabinofurano-alkyne to iminogalactitol-derived nitrone. Remarkably, the nucleophilic addition could be performed highly efficiently and stereoselectively in the presence of diethylzinc in toluene using protected nitrone and alkynyl sugar as reaction partners and thus opens a concise approach to a diversity of disaccharide mimics. (C) 2012 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
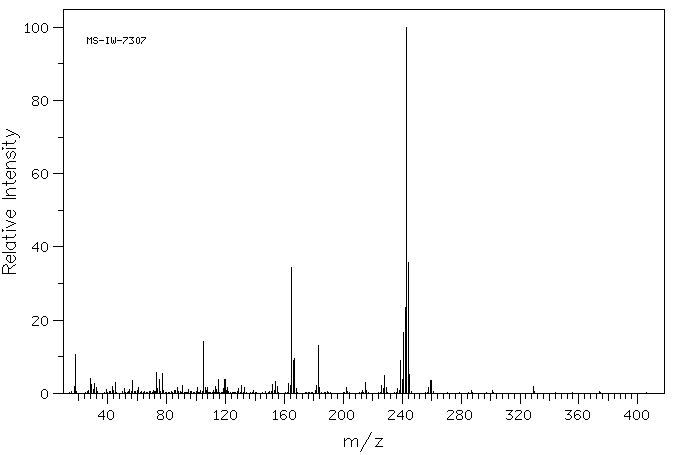
质谱MS
-
碳谱13CNMR
-
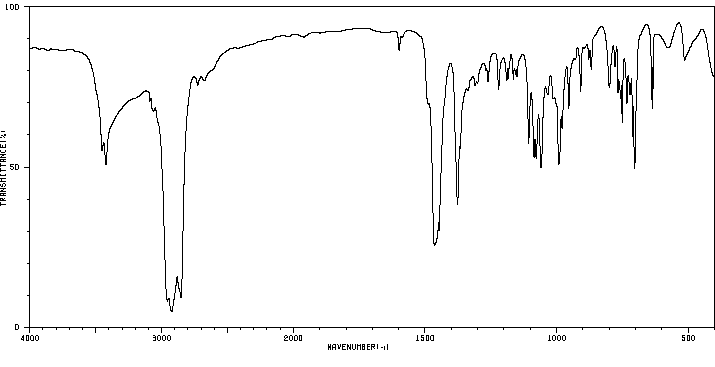
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-三苯基甲氨基甲基)吡啶
非马沙坦杂质1
隐色甲紫-d6
隐色孔雀绿-d6
隐色孔雀绿
隐色乙基结晶紫
降钙素杂质10
重氮四苯基乙烷
酸性黄117
酸性蓝119
酚酞啉
酚酞二硫酸钾水合物
萘,1-甲氧基-3-甲基
苯酚,4-(1,1-二苯基丙基)-
苯甲醇,4-溴-a-(4-溴苯基)-a-苯基-
苯甲醇,2-氨基-5-氯-a-乙烯基-a-苯基-
苯甲酸,4-(羟基二苯甲基)-,甲基酯
苯甲酸,3-[[2-[[(1,1-二甲基乙氧基)羰基]氨基]-3-[(三苯代甲基)硫代]丙基]氨基]-,(R)-
苯甲基N-[(2(三苯代甲基四唑-5-基-1,1联苯基-4-基]-甲基-2-氨基-3-甲基丁酸酯
苯基双-(对二乙氨基苯)甲烷
苯基二甲苯基甲烷
苯基二[2-甲基-4-(二乙基氨基)苯基]甲烷
苯基{二[4-(三氟甲基)苯基]}甲醇
苯基-二(2-羟基-5-氯苯基)甲烷
苄基2,3,4-三-O-苄基-6-O-三苯甲基-BETA-D-吡喃葡萄糖苷
苄基 5-氨基-5-脱氧-2,3-O-异亚丙基-6-O-三苯甲基呋喃己糖苷
苄基 2-乙酰氨基-2-脱氧-6-O-三苯基-甲基-alpha-D-吡喃葡萄糖苷
苄基 2,3-O-异亚丙基-6-三苯甲基-alpha-D-甘露呋喃糖
苄基 2,3,4-三-O-(苯基甲基)-6-O-(三苯基甲基)-ALPHA-D-吡喃甘露糖苷
芴甲氧羰基-4-叔丁酯-天冬酰胺-S-三氯苯甲基-L-半胱氨酸
膦酸,1,2-乙二基二(磷羧基甲基)亚氨基-3,1-丙二基次氮基<三价氮基>二(亚甲基)四-,盐钠
脱氢奥美沙坦-2三苯甲基奥美沙坦脂
美托咪定杂质28
绿茶提取物茶多酚陕西龙孚
结晶紫
磺基琥珀酰亚胺基-4-[2-(4,4-二甲氧基三苯甲基)]丁酸酯
磷,三(4-甲氧苯基)甲基-,碘化
碱性蓝
硫代硫酸氢 S-[2-[(3,3,3-三苯基丙基)氨基]乙基]酯
盐酸三苯甲基肼
白孔雀石绿-d5
甲酮,(反-4-氨基-4-甲基环己基)-4-吗啉基-
甲基三苯基甲基醚
甲基6-O-(三苯基甲基)-ALPHA-D-吡喃甘露糖苷三苯甲酸酯
甲基3,4-O-异亚丙基-6-O-三苯甲基-beta-D-吡喃半乳糖苷
甲基3,4-O-异亚丙基-2-O-甲基-6-O-三苯甲基吡喃己糖苷
甲基2-甲基-N-{[4-(三氟甲基)苯基]氨基甲酰}丙氨酸酸酯
甲基2,3,4-三-O-苯甲酰基-6-O-三苯甲基-ALPHA-D-吡喃葡萄糖苷
甲基2,3,4-三-O-苄基-6-O-三苯甲基-ALPHA-D-吡喃葡萄糖苷
甲基2,3,4-三-O-(苯基甲基)-6-O-(三苯基甲基)-ALPHA-D-吡喃半乳糖苷