1,3,5-tris-(4-chloro-phenyl)-[1,3,5]triazinane-2,4,6-trione | 1784-98-1
中文名称
——
中文别名
——
英文名称
1,3,5-tris-(4-chloro-phenyl)-[1,3,5]triazinane-2,4,6-trione
英文别名
1,3,5-Tris(4-chlorophenyl)-1,3,5-triazinane-2,4,6-trione
CAS
1784-98-1
化学式
C21H12Cl3N3O3
mdl
——
分子量
460.704
InChiKey
KSDJQIFGRDGQJC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:30
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:60.9
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为产物:参考文献:名称:硅酰胺基配体支撑的二烷基钇:异氰酸酯环三聚的合成,结构和催化† ‡摘要:合成了空间需求的硅sil(ArN = Si(L)NHAr)配体,并用于制备钇二烷基络合物,该催化剂催化了异氰酸酯的环三聚反应,具有很高的活性和出色的官能团耐受性。DOI:10.1039/c9cc06282c
文献信息
-
Synergistic Catalysis by Brønsted Acid/Carbodicarbene Mimicking Frustrated Lewis Pair‐Like Reactivity作者:Yi‐Chen Chan、Yuna Bai、Wen‐Ching Chen、Hsing‐Yin Chen、Chen‐Yu Li、Ying‐Yann Wu、Mei‐Chun Tseng、Glenn P. A. Yap、Lili Zhao、Hsuan‐Ying Chen、Tiow‐Gan OngDOI:10.1002/anie.202107127日期:2021.9Carbodicarbene (CDC), unique carbenic entities bearing two lone pairs of electrons are well-known for their strong Lewis basicity. We demonstrate herein, upon introducing a weak Brønsted acid benzyl alcohol (BnOH) as a co-modulator, CDC is remolded into a Frustrated Lewis Pair (FLP)-like reactivity. DFT calculation and experimental evidence show BnOH loosely interacting with the binding pocket of CDC碳二碳烯 (CDC) 是一种独特的碳烯实体,带有两对孤对电子,以其强路易斯碱度而闻名。我们在此证明,在引入弱 Brønsted 酸苄醇 (BnOH) 作为共调节剂后,CDC 被重塑为类似受挫路易斯对 (FLP) 的反应性。DFT 计算和实验证据表明,BnOH 通过 H 键合和 π-π 堆积与 CDC 的结合口袋松散地相互作用。部署了四种不同的自然界反应,以证明作为协同 FLP/调节剂 (CDC/BnOH) 的概念验证的可行性,证明异氰酸酯环三聚、L聚合过程中的催化反应性增强-丙交酯 (LA)、甲基丙烯酸甲酯 (MMA) 和醇的脱氢硅烷化。重要的是,碳二碳烯的催化反应性与仅依赖于亲核性的单一化学特征的常规 NHC 截然不同。这一发现还为通过共调节剂或助催化剂使 FLP 反应性多样化提供了新的思路。
-
The Synthesis of Isocyanurates on the Trimerization of Isocyanates under High Pressure作者:Yoichi Taguchi、Isao Shibuya、Masahiko Yasumoto、Tohru Tsuchiya、Katsumi YonemotoDOI:10.1246/bcsj.63.3486日期:1990.12The trimerization of phenyl isocyanate in the presence of triethylamine was accelerated under high pressure to give triphenyl isocyanurate almost quantitatively. The reaction in benzene was remarkably accelerated by compression. The effects of pressure, temperature, catalysts, and solvents were examined on the trimerization of phenyl isocyanate. Aryl and normal alkyl isocyanates trimerized under high
-
An Electron-Rich Proazaphosphatrane for Isocyanate Trimerization to Isocyanurates作者:Steven M. Raders、John G. VerkadeDOI:10.1021/jo9023396日期:2010.8.6synthesis of the new electron-rich, sterically hindered proazaphosphatrane shown above is described herein. This proazaphosphatrane catalyzes the cyclotrimerization of a wide variety of isocyanates to isocyanurates under mild conditions with unprecedentedly fast reaction times, giving moderate to high product yields. It is also shown that this proazaphosphatrane can be recycled up to 5 times.
-
First Synthesis of a Highly Basic Dendrimer and its Catalytic Application in Organic Methodology作者:Arunkanti Sarkar、Palanichamy Ilankumaran、Philip Kisanga、John G. VerkadeDOI:10.1002/adsc.200404089日期:2004.8Sixteen OCH2CH2N3P(i-BuNCH2CH2)3N substituents (A) containing the highly basic bicyclic azido-phosphine moiety shown, have been incorporated into the dendrimer [CH2CH2N[(CH2)3N[(CH2)3NHC(O)-3,5-bis-(A)-C6H3]2]2]2 in five steps from commercially available starting materials with an overall yield of 22.5%. Also presented are examples of Michael additions, nitroaldol reactions and aryl isocyanate trimerizations
-
Cyclic tetramers of a five-membered palladacycle based on a head-to-tail-linked isocyanate dimer and their reactivity in cyclotrimerization of isocyanates作者:Seon Gye Lee、Keun-Young Choi、Yong-Joo Kim、SuJin Park、Soon W. LeeDOI:10.1039/c5dt00534e日期:——Reactions of [Pd(styrene)(PR3)2], generated from trans-[PdEt2(PR3)2] and styrene, with 2 equiv. of benzyl isocyanate in THF at room-temperature afforded unusual cyclic Pd-tetramers of five-membered rings consisting of organic isocyanate dimers and palladium, [Pd(PR3)–C(O)N(R)C(O)N(R)–}]4 (PR3 = PMe3, 1; PR3 = PMe2Ph, 2). Additionally, a cyclic trimer, (RNCO)3, 3 (R = benzyl) was produced as a catalytic的反应[钯(苯乙烯)(PR 3)2 ],从生成的反式- [PDET 2(PR 3)2 ]和苯乙烯,与2当量 室温下将异氰酸苄酯在THF中制得的五元环的非常规环状Pd-四聚体由有机异氰酸酯二聚体和钯组成,[Pd(PR 3)– C(O)N(R)C(O)N( R) - }] 4(PR 3 = PME 3,1 ; PR 3 = PME 2 PH,2)。另外,环状三聚体,(RNCO)3,3(R =苄基)作为催化产物产生。用4当量处理环状四聚体(1)。(1,2-双(二乙基膦基)乙烷)(DEPE)或(1,2-双(二甲基膦基)乙烷)(DMPE)等螯合膦容易引起金属环顺式[Pd N (R)C(O)N(R)C(O)}(P〜P)](P〜P = DEPE,4 ; P〜P = DMPE,5)以定量产率计算。相反,Pd(0)-PR 3与2当量的反应。N- CO (Ar = Ph,p - tolyl ,p -ClC
表征谱图
-
氢谱1HNMR
-
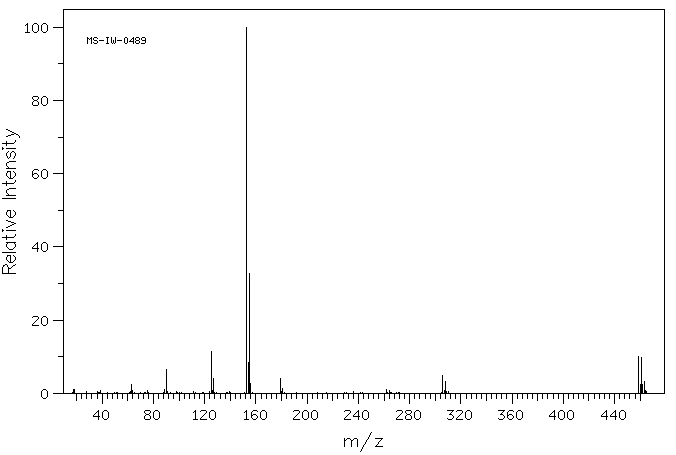
质谱MS
-
碳谱13CNMR
-
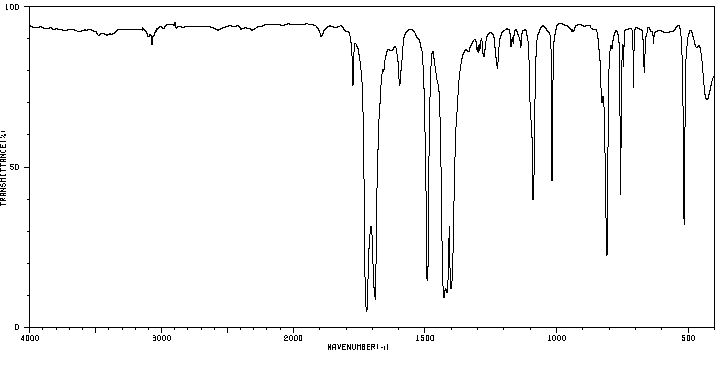
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4