3-叔-丁基-2-吡唑啉-5-酮 | 29211-68-5
中文名称
3-叔-丁基-2-吡唑啉-5-酮
中文别名
——
英文名称
3-tert-butyl-1H-pyrazol-5(4H)-one
英文别名
5-tert-butyl-2,4-dihydro-3H-pyrazol-3-one;3-tert.-Butyl-pyrazolon-(5);3-tert-Butyl-2-pyrazolin-5-one;3-tert-butyl-1,4-dihydropyrazol-5-one
CAS
29211-68-5
化学式
C7H12N2O
mdl
MFCD00051760
分子量
140.185
InChiKey
VDNOHRWEOSDGQX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:208 °C
-
密度:1.11±0.1 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:41.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2933199090
-
安全说明:S22,S24/25
SDS
| Name: | 3-(tert-Butyl)-4 5-dihydro-1H-pyrazol-5-one TECH Material Safety Data Sheet |
| Synonym: | 3-tert-Butyl-2-pyrazoline-5-on |
| CAS: | 29211-68-5 |
Synonym:3-tert-Butyl-2-pyrazoline-5-on
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 29211-68-5 | 3-(tert-Butyl)-4,5-dihydro-1H-pyrazol- | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 29211-68-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 208 - 210 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H12N2O
Molecular Weight: 140.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 29211-68-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-(tert-Butyl)-4,5-dihydro-1H-pyrazol-5-one - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 29211-68-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 29211-68-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 29211-68-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:3-溴邻苯二酚 、 3-叔-丁基-2-吡唑啉-5-酮 在 laccase from Agaricus bisporus 作用下, 以 aq. phosphate buffer 为溶剂, 反应 17.0h, 以96%的产率得到3'-bromo-4'-(3-tert-butyl-5-hydroxy-1H-pyrazol-4-yl)benzene-1',2'-diol参考文献:名称:Laccase-catalyzed reaction of 3-tert-butyl-1H-pyrazol-5(4H)-one with substituted catechols using air as an oxidant摘要:The laccase-catalyzed reaction of 3-tert-butyl-1H-pyrazol-5(4H)-one with a number of catechols and aerial oxygen as an oxidant selectively affords 4-substituted 3-tert-butyl-1H-pyrazol-5-ol derivatives with yields ranging from 77 to 99%. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2013.03.023
文献信息
-
Substituted Imidazopyridines as HDM2 Inhibitors申请人:Merck Sharp & Dohme Corp.公开号:US20140179680A1公开(公告)日:2014-06-26The present invention provides substituted imidazopyridines as described herein or a pharmaceutically acceptable salt or solvate thereof. The representative compounds are useful as inhibitors of the HDM2 protein. Also disclosed are pharmaceutical compositions comprising the above compounds and potential methods of treating cancer using the same.
-
NEPRILYSIN INHIBITORS申请人:Fleury Melissa公开号:US20150210690A1公开(公告)日:2015-07-30In one aspect, the invention relates to compounds having the formula I: where R 1 -R 6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. These compounds have neprilysin inhibition activity. In another aspect, the invention relates to pharmaceutical compositions comprising these compounds; methods of using these compounds; and processes and intermediates for preparing these compounds.在一个方面,该发明涉及具有以下式I的化合物: 其中R1-R6如规范中定义,或其药学上可接受的盐。这些化合物具有神经肽酶抑制活性。在另一个方面,该发明涉及包含这些化合物的药物组合物;使用这些化合物的方法;以及制备这些化合物的过程和中间体。
-
METHOD OF TREATING CONTRAST-INDUCED NEPHROPATHY申请人:FOO Shi Yin公开号:US20120122844A1公开(公告)日:2012-05-17The present invention provides the use of a neutral endopeptidase inhibitor, in the manufacture of a medicament for the treatment, amelioration and/or prevention of contrast-induced nephropathy. The invention also relates to the use of a compound of Formula I: wherein R 1 , R 2 , R 3 , R 5 , X, A 3 , B 1 , s and n are defined herein, for the treatment, amelioration and/or prevention of contrast-induced nephropathy. The present invention further provides a combination of pharmacologically active agents for use in the treatment, amelioration and/or prevention of contrast-induced nephropathy.本发明提供了中性内切肽酶抑制剂的使用,用于制造用于治疗、改善和/或预防造影剂诱导性肾病的药物。该发明还涉及使用化合物Formula I中的化合物:其中R1、R2、R3、R5、X、A3、B1、s和n在此处定义,用于治疗、改善和/或预防造影剂诱导性肾病。本发明进一步提供了用于治疗、改善和/或预防造影剂诱导性肾病的药理活性剂的组合。
-
Versatile three-component synthesis of 2′-amino-1,2-dihydrospiro[(3H)-indole-3,4′-(4′H)-pyran]-2-ones作者:V. Yu. Mortikov、Yu. M. Litvinov、A. A. Shestopalov、L. A. Rodinovskaya、A. M. ShestopalovDOI:10.1007/s11172-008-0338-7日期:2008.112-dihydrospiro[(3H)-indole-3,4′-(4′H)-pyran]-2-ones has been suggested consisting in the three-component reaction of isatins, cyanoacetic acid derivatives, and α-methylenecarbonyl compounds (β-dicarbonyl compounds, activated phenols, and OH-substituted heterocycles) in ethanol in the presence of triethylamine as a catalyst. The reaction proceeds selectively to form spiro[(3H)-indole-3,4′-(4′H)-pyrans].
-
Synthesis of heterocyclic compounds from 4-formylpyrazoles作者:V. Yu. Mortikov、L. A. Rodinovskaya、A. E. Fedorov、A. M. Shestopalov、P. A. BelyakovDOI:10.1007/s11172-014-0451-8日期:2014.2Formylation of pyrazole and 2,5-dimethylpyrazole gave a number of pyrazole-containing aldehydes, which can be used to obtain chromenes, tetrahydrochromenes, 1,4-dihydropyrano[2,3-c]pyrazoles, pyrano[3,2-c]chromenes, thiochromeno[4,3-b]pyrans, pyrano[3,2-c]-quinolines, and thiazolo[3,2-a]pyridines.
表征谱图
-
氢谱1HNMR
-
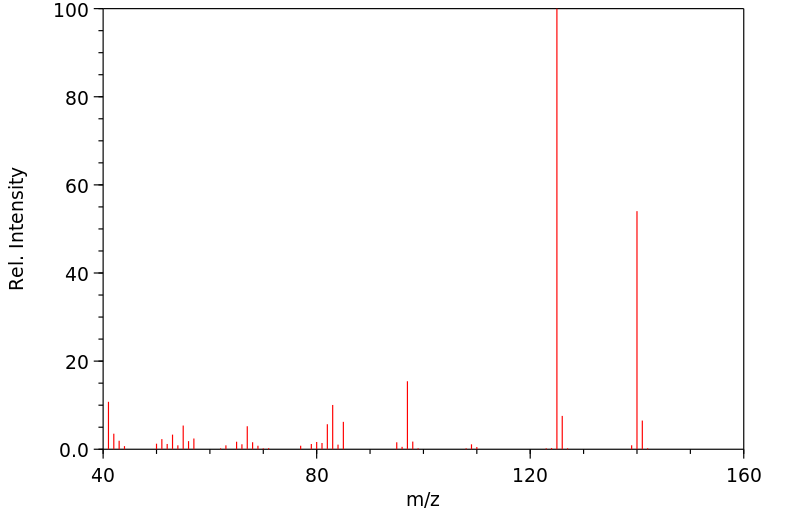
质谱MS
-
碳谱13CNMR
-
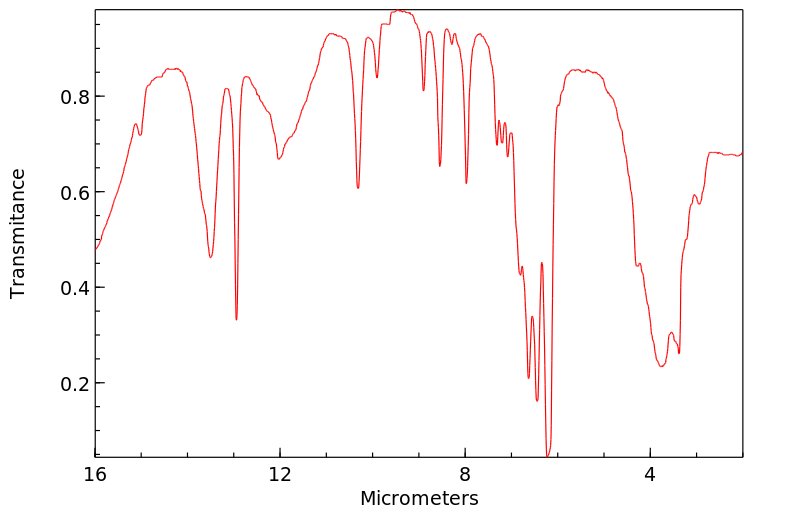
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4S,4''S)-2,2''-环亚丙基双[4-叔丁基-4,5-二氢恶唑]
香豆素-6-羧酸
顺式-3a,5,6,6a-四氢-3-(1-甲基乙基)-4H-环戊二烯并[d]异恶唑
锌离子载体IV
钐(III) 离子载体 II
苯,1-(2E)-2-丁烯-1-基-2-氟-
苯,(2,2-二氟乙烯基)-
聚二硫二噻唑烷
缩胆囊肽9
绕丹酸钠
盐(1:?)5'-尿苷酸,钠
甲酰乙内脲
甲巯咪唑
甲基羟甲基油基噁唑啉
甲基5-羟基-3,5-二甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-氰基-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基5-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-(羟基甲基)-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基4-甲基-5-氧代-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4,5-二氮杂螺[2.4]庚-5-烯-6-羧酸酯
甲基4,5-二氢-5-乙基-1H-吡唑-1-羧酸酯
甲基3-甲基-4,5-二氢-1,2-恶唑-4-羧酸酯
甲基(E)-3-[6-[1-羟基-1-(4-甲基苯基)-3-(1-吡咯烷基)丙基]-2-吡啶基]丙烯酰酸酯
甲基(5-氧代-4,5-二氢-1,2-恶唑-3-基)乙酸酯
环戊二烯并[d]咪唑-2,5(1H,3H)-二硫酮
环己羧酸,3-氨基-2-甲氧基-,甲基酯,(1S,2S,3S)-
溶剂黄93
溴化1-十六烷基-3-甲基咪唑
溴化1-十二烷基-2,3-二甲基咪唑
泰比培南酯中间体
泰比培南酯中间体
氨甲酸,[4,5-二氢-4-(碘甲基)-2-噻唑基]-,1,1-二甲基乙基酯(9CI)
氨基甲硫酸,[2-[[(2-羰基-1-咪唑烷基)硫代甲基]氨基]乙基]-,O-甲基酯
异噻唑,4,5-二氯-2,5-二氢-2-辛基-
希诺米啉
四氟硼酸二氢1,3-二(叔-丁基)-4,5--1H-咪唑正离子
四唑硝基紫
噻唑烷-2,4-二酮-2-缩氨基脲
噻唑丁炎酮
噻唑,4,5-二氢-4-(1-甲基乙基)-,(S)-
噁唑,4,5-二氢-4,4-二甲基-2-(5-甲基-2-呋喃基)-
噁唑,2-庚基-4,5-二氢-
咪唑烷基脲
吡嗪,2,3-二氢-5,6-二甲基-2-丙基-
叔-丁基3-羟基-1,4,6,7-四氢吡唑并[4,3-c]吡啶-5-羧酸酯
双吡唑啉酮