2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯 | 50708-37-7
中文名称
2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯
中文别名
——
英文名称
tetramethyltetrathiafulvalene
英文别名
TMTTF;2-(4,5-dimethyl-1,3-dithiol-2-ylidene)-4,5-dimethyl-1,3-dithiole
CAS
50708-37-7
化学式
C10H12S4
mdl
——
分子量
260.469
InChiKey
HGOTVGUTJPNVDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:14
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:101
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2930909090
SDS
上下游信息
反应信息
-
作为反应物:描述:2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯 在 2,6-dichlorobenzenediazonium 2-nitro-1,3-indanedionate 作用下, 以 四氯化碳 为溶剂, 反应 0.28h, 以85%的产率得到tetramethyltetrathiafulvalene 2-nitro-1,3-indanedionate参考文献:名称:Kokars, V. R.; Kampar, V. E.; Neiland, O. Ya., Journal of Organic Chemistry USSR (English Translation), 1983, p. 1092 - 1095摘要:DOI:
-
作为产物:描述:2-methoxy-4,5-dimethyl-1,3-dithiole 在 三氯乙酸 作用下, 以 苯 为溶剂, 反应 1.0h, 以95%的产率得到2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯参考文献:名称:Buza, Daniela; Gradowska, Wanda, Polish Journal of Chemistry, 1984, vol. 58, # 7-8-9, p. 775 - 782摘要:DOI:
-
作为试剂:描述:4-(N,N-diethylamino)benzenediazonium tetrafluoroborate 在 2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯 作用下, 以 乙腈 为溶剂, 生成参考文献:名称:Kampar, V. E.; Bumbure, G. V.; Kokars, V. R., Journal of general chemistry of the USSR, 1980, vol. 50, # 9, p. 1663 - 1666摘要:DOI:
文献信息
-
Spectroscopic and magnetic properties of FeOCl intercalated with organosulfur electron donors作者:S. M. Kauzlarich、J. F. Ellena、P. D. Stupik、W. M. Rieff、B. A. AverillDOI:10.1021/ja00249a019日期:1987.7conductor, the intercalation chemistry of FeOCl with tetrathiolene molecules has been explored. New intercalation compounds of the organic electron donors TTF (tetrathiafulvalene), TMTTF (tetramethyltetrathiafulvalene), TTN (tetrathianaphthalene), and TTT (tetrathiatetracene) with the inorganic host FeOCl were prepared by direct reaction of solutions of the organosulfur compounds with solid FeOCl. The new为了开发一种新型的低维导体,已经探索了 FeOCl 与四硫醇分子的嵌入化学。有机电子给体 TTF(四硫富瓦烯)、TMTTF(四甲基四硫富瓦烯)、TTN(四硫杂萘)和 TTT(四硫杂蒽)与无机主体 FeOCl 的新型插层化合物是通过有机硫化合物溶液与固体 FeOCl 的直接反应制备的。获得的新相为 FeOCl(TTF)/sub 1/8.5/、FeOCl(TMTTF)/sub 1/13/、FeOCl(TTN)/sub 1/9/(tol)/sub 1/22/ 和 FeOCl( TTT)/sub 1/9/ (tol)/sub 1/23/。X 射线粉末衍射数据与四硫醇分子垂直于层取向一致,但 FeOCl(TMTTF)/sub 1/13/ 除外,其中 TMTTF 分子平行于主体层取向。对 FeOCl(TTF)/sub 1/8.5/ 和相关模型化合物进行的详细宽线 /sup 1/H 和交叉极化魔角旋转 (CPMAS)
-
An ESR Study of the Radical Cations of Tetrathiafulvalene (TTF) and Electron Donors Containing the TTF Moiety作者:Luka ?avara、Fabian Gerson、Dwaine O. Cowan、Knud LerstrupDOI:10.1002/hlca.19860690117日期:1986.2.5Hyperfine data and g factors are reported for the radical cations of tetrathiafulvalene (TTF; 1) and of its derivatives 2–13. From the intense satellite spectra of 1+–13+ not only the coupling constants of the 33S isotopes in the TTF moiety could be determined, but also, in favourable cases, those of the 13C isotopes in the central double bond. The former values range from 0.370 (8+) to 0.470 mT (4+) and the报道了四硫富瓦烯(TTF; 1)及其衍生物2-13的自由基阳离子的超精细数据和g因子。从1 + – 13 +的强卫星光谱中,不仅可以确定TTF部分中33 S同位素的偶合常数,而且在有利的情况下,还可以确定中心双键中13 C同位素的偶合常数。前者的值范围为0.370(8 +)至0.470 mT(4 +),而后者的值范围为0.255(8 +)至0.360 mT(4 +))在二的自由基阳离子(亚乙基)-TTF(8 +)和四氰基-TTF(4 +)。TTF的自由基阳离子(1 +)显示出中间值,为0.425的33 S和0.285 mT的为13个Ç同位素。1 + – 13 +中的自旋种群在很大程度上位于π系统的中央S 2 C CS 2部分。它倾向于通过在TTF的2、3、6、7位上被电子接受(供体)基团取代而增加(减少)。
-
New Molecular Charge‐Transfer Salts of TM‐TTF and BMDT‐TTF with Thiocyanate and Selenocyanate Complex Anions [TMTTF = Tetramethyltetrathiafulvalene; BMDT‐TTF = Bis(methylenedithio)tetrathiafulvalene]作者:Marta Mas‐Torrent、Scott S. Turner、Klaus Wurst、José Vidal‐Gancedo、Jaume Veciana、Peter Day、Concepció RoviraDOI:10.1002/ejic.200390099日期:2003.2Three new charge-transfer salts of tetrathiafulvalene (TTF)-based donors with thiocyanato- or selenocyanato-metal complex anions have been synthesised. The salts isolated were [BMDT-TTF]4[Cr(NCS)6] (1), [TM-TTF]4[Cr(NCS)6]·2CH3CN (2) and [TM-TTF]4[Cr(NCSe)6]·2CH3CN (3) [BMDT-TTF = bis(methylenedithio)tetrathiafulvalene and TM-TTF = tetramethyltetrathiafulvalene]. Single crystals of compound 1 crystallise已经合成了三种新的四硫富瓦烯 (TTF) 基供体与硫氰酸根合或硒氰酸根合金属络合阴离子的电荷转移盐。分离出的盐为 [BMDT-TTF]4[Cr(NCS)6] (1), [TM-TTF]4[Cr(NCS)6]·2CH3CN (2) 和 [TM-TTF]4[Cr(NCS) )6]·2CH3CN (3) [BMDT-TTF = 双(亚甲基二硫基)四硫富瓦烯和 TM-TTF = 四甲基四硫富瓦烯]。化合物 1 的单晶在单斜 C2/c 空间群中结晶,a = 37.286(3), b = 10.0539(6), c = 21.069(2) A, β = 124.348(4)°, V = 6520.9(9) ) A3 和 Z = 4。化合物 3 也适用于 X 射线衍射研究,但是阴离子部分 [Cr(NCSe)6]- 高度无序,最佳溶液的最终 R 因子为 16.4%。找到了单斜空间群 C2/m 的解,a =
-
Synthesis of unsymmetrical tetrathiafulvalenes and electrical conductivities of their tetracyanoquinodimethane complexes作者:Hitoshi Tatemitsu、Etsushi Nishikawa、Yoshiteru Sakata、Soichi MisumiDOI:10.1039/c39850000106日期:——The preparation of some unsymmetrical tetrathiafulvalenes and the electrical conductivities of their charge transfer complexes with tetracyanoquinodimethane (TCNQ) are described; the ethylenedithio(trimethylene)tetrathiafulvalene–TCNQ complex exhibited higher conductivity than complexes of tetrathiafulvalene, tetramethyltetrathia-fulvalene, and hexamethylenetetrathiafulvalene with TCNQ.
-
Three-Dimensionally-Modified Tetracyanoquinodimethanes and Their Charge-Transfer Complexes with Tetrathiafulvalene Derivatives Having a Wide Range of Ionicity作者:Kazuhiro Nakasuji、Masakatsu Nakatsuka、Hideki Yamochi、Ichiro Murata、Shigeharu Harada、Nobutami Kasai、Kimiaki Yamamura、Jiro Tanaka、Gunzi Saito、Toshiaki Enoki、Hiroo InokuchiDOI:10.1246/bcsj.59.207日期:1986.1Eight different 1:1 charge-transfer(CT) complexes were prepared using tetrahydrobarreleno-tetracyanoquinodimethane(THBTCNQ), dihydrobarreleno-tetracyanoquinodimethane(DHBTCNQ), monobenzobarreleno-tetracyanoquinodimethane(MBBTCNQ), and dibenzobarreleno-tetracyanoquinodimethane(DBBTCNQ) as tetracyanoquinodimethane(TCNQ)-type acceptors, and tetrathiafulvalene(TTF), bis(tetramethyene)-tetrathiafulvalene(OMTTF), and tetramethyl-tetrathiafulvalene(TMTTF) as TTF-type donors. The complexes can be classified into three distinct groups, I–III, on the basis of the magnitude of both the degree of CT and the electrical resistivity of compacted powders. Group-I complexes are characterized by a small band gap semiconductivity and moderate CT, group-II complexes by low conductivity and small CT, and group III complexes by low conductivity and complete CT. The stacking mode of the three groups of complexes are discussed, by comparing the electronic absorption spectra of the three groups of CT complexes with those of the complexes for which the crystal structures are known. A crystal structure analysis of OMTTF–DBBTCNQ revealed that it is the first example of a highly ionic CT complex of a TTF–TCNQ type with a regular mixed stacking mode. These three groups of complexes containing bulky acceptor groups will be of theoretical interest in studying the crystal properties of organic CT complexes.制备了八种不同的1:1电荷转移(CT)复合物,使用四氢巴伦烯-四氰基喹啉二甲烷(THBTCNQ)、二氢巴伦烯-四氰基喹啉二甲烷(DHBTCNQ)、单苯巴伦烯-四氰基喹啉二甲烷(MBBTCNQ)和二苯巴伦烯-四氰基喹啉二甲烷(DBBTCNQ)作为TCNQ型受体,四硫富瓦烯(TTF)、双(四甲烯)-四硫富瓦烯(OMTTF)和四甲基-四硫富瓦烯(TMTTF)作为TTF型施体。这些复合物可根据CT程度和压缩粉末的电阻率大小分为三组:I–III。I组复合物具有小带隙半导体特性和中等的CT,II组复合物表现为低导电性和小CT,而III组复合物则具有低导电性和完全CT。通过比较这三组CT复合物的电子吸收光谱与已知晶体结构复合物的光谱,讨论了这三组复合物的堆叠模式。对OMTTF–DBBTCNQ的晶体结构分析表明,它是第一例具有规则混合堆叠模式的高离子CT复合物,属于TTF–TCNQ类型。这三组含有庞大受体基团的复合物在研究有机CT复合物的晶体特性方面具有理论意义。
表征谱图
-
氢谱1HNMR
-
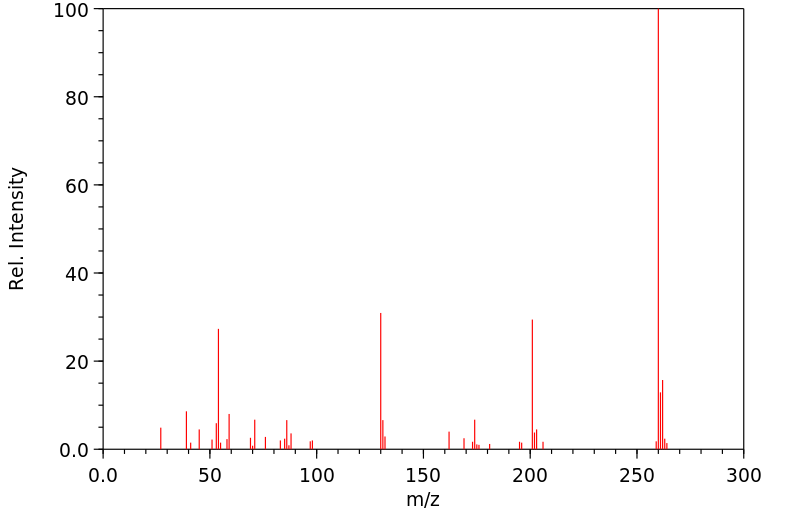
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
四硫杂富瓦烯-D4
四硫富瓦烯
四(戊硫代)四硫富瓦烯
四(十八烷基硫代)四硫富瓦烯
四(乙硫基)四硫富瓦烯[有机电子材料]
双(亚乙基二硫醇)四硫代富瓦烯
双(三亚甲基二硫代)四硫富瓦烯
三(四硫富瓦烯)双(四氟硼酸盐)复合物
[1,3]二噻唑并[4,5-d]-1,3-二噻唑,2,5-二(1,3-二硫醇-2-亚基)-
5-甲基二硫杂环戊烯-3-硫酮
5-氨基-3-硫代氧基-3H-(1,2)二硫杂环戊烯-4-羧酸乙酯
5-氨基-3-硫代氧基-3H-(1,2)二硫杂环戊烯-4-甲腈
5,6-二氢-4H-环戊并[1,2]二硫代-3-硫酮
4,4’,5-三甲基四硫富瓦烯
4-甲基二硫杂环戊烯-3-硫酮
4-新戊基-3H-1,2-二硫杂环戊烯-3-硫酮
4,5-二甲基-3H-1,2-二硫醇-3-酮
4,5,6,7-四氢苯并[1,2]二硫-3-硫酮
4,4’-二甲基连四硫富瓦烯
4,4,5,5,6,6,7,7-八氢二苯并四硫富瓦烯
3H-1,2-二硫杂环戊二烯-3-酮
3H-1,2-二硫杂环戊二烯-3-硫酮
2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯
2,3,6,7-四(2-氰乙基硫代)四硫富瓦烯
1,3-二噻唑,2-[4,5-二(癸基硫代)-1,3-二硫醇-2-亚基]-4,5-二(癸基硫代)-
1,3-二噻唑,2-[4,5-二(十四烷基硫代)-1,3-二硫醇-2-亚基]-4,5-二(十四烷基硫代)-
1,3-二噻唑,2-[4,5-二(十一烷基硫代)-1,3-二硫醇-2-亚基]-4,5-二(十一烷基硫代)-
(四甲基硫)四硫富瓦烯
3-[[2-[4,5-Bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-(2-cyanoethylsulfanyl)-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propanenitrile
4,5-Bis-{2-[2-(2-iodo-ethoxy)-ethoxy]-ethylsulfanyl}-4',5'-bis-methylsulfanyl-[2,2']bi[[1,3]dithiolylidene]
2-<4,5-bis(methylthio)-1,3-dithiol-2-ylidene>-5-(thiopyran-4-ylidene)-1,3,4,6-tetrathiapentalene
2,3-bis(2-cyanoethylthio)-6,7-bis(2-hydroxyethylthio)tetrathiafulvalene
4,5-bis(decylthio)-4'-(3-cyanopropyl)thio-5-methyltetrathiafulvalene
4,5,4',5'-Tetrakis-trimethylsilanylethynyl-[2,2']bi[[1,3]dithiolylidene]
bis(Dimethylvinylenedithio)tetrathiafulvalene
2,3-Bis{2-[2-(2-chloroethoxy)ethoxy]ethylthio}-6-(2-cyanoethylthio)-7-methylthiotetrathiafulvalene
3-[5-(2-Cyano-ethylselanyl)-2-methylsulfanyl-[1,3]dithiol-4-ylselanyl]-propionitrile
2-(4-Pent-4-ynyl-[1,3]dithiol-2-ylidene)-5,6-dihydro-[1,3]dithiolo[4,5-b][1,4]dithiine
2-(4-Nonadeca-4,6-diynyl-[1,3]dithiol-2-ylidene)-5,6-dihydro-[1,3]dithiolo[4,5-b][1,4]dithiine
5-Trifluoromethyl-[1,2]dithiole-3-thione
4-[(trimethylsilyl)ethynyl]-5-methyl-4',5'-ethylenedithiotetrathiafulvalene
[4-Methyl-5-methylsulfanyl-[1,2]dithiol-(3Z)-ylidene]-thioacetic acid S-methyl ester
1,3-Dithiolo[4,5-b][1,4]dithiin,5,6-dihydro-2-[4-(9-decynyl)-1,3-dithiol-2-ylidene]-
di(vinylthio)ethylenedithiotetrathiafulvalene
2,3:8,9-Bis(ethylendithio)-1,4,7,10-tetrathiafulvalen, CT-Komplex mit 2,5-Bis(cyanimino)-2,5-dihydro-3,6-diiodthieno<3,2-b>thiophen
4-ethyl-2-isopropylidene-[1,3]dithiole
2-[1-Chloro-1-methylsulfanylcarbonyl-meth-(Z)-ylidene]-5-methylsulfanyl-[1,3]dithiole-4-carbothioic acid S-methyl ester
tetra(vinylthio)tetrathiafulvalene