2,4,6-三乙基-1,3,5-三氧杂环己烷 | 2396-42-1
中文名称
2,4,6-三乙基-1,3,5-三氧杂环己烷
中文别名
——
英文名称
2,4,6-Triethyl-[1,3,5]trioxane
英文别名
2,4,6-Triethyl-1,3,5-trioxan;2,4,6-Triethyl-1,3,5-trioxane
CAS
2396-42-1
化学式
C9H18O3
mdl
MFCD01677648
分子量
174.24
InChiKey
GYBGKXFOKOSPLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:57-57.5 °C(Press: 10 Torr)
-
密度:0.9418 g/cm3(Temp: 22 °C)
-
物理描述:Liquid
-
熔点:-20°C
-
保留指数:1285;1254
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三聚乙醛 paracetaldehyde 123-63-7 C6H12O3 132.159
反应信息
-
作为反应物:描述:2,4,6-三乙基-1,3,5-三氧杂环己烷 、 溴 生成 2,2-dibromo-propionaldehyde diethylacetal 、 2-溴呋喃丙醛二甲缩醛 、 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:Dworzak; Pfifferling, Monatshefte fur Chemie, 1927, vol. 48, p. 252摘要:DOI:
-
作为产物:描述:丙醛 在 N,N,N',N'',N''-pentamethyl-N,N''-bis(3-sulfopropyl)diethylenetriaminium tris(trifluoromethanesulfonate) 作用下, 以 neat (no solvent) 为溶剂, 反应 1.5h, 以91.3%的产率得到2,4,6-三乙基-1,3,5-三氧杂环己烷参考文献:名称:在室温下无溶剂条件下合成1,3,5-三恶烷的简便方法摘要:摘要以脂肪族多胺和1,3-丙烷磺酸为原料,合成了一系列对环境有益的双-SO 3 H-官能化的布朗斯台德酸性离子液体。这些离子液体在室温下在无溶剂条件下充当脂族醛环三聚的有效廉价且可回收的催化剂。反应进行顺利,分离产率良好至优异(66.9-97.6%=),当离子液体的量为0.1 mol%时,反应通常在1.5小时内完成。离子液体可以容易地回收并重复使用五次,而其催化活性没有任何重大损失。 图形概要DOI:10.1007/s00706-014-1150-8
文献信息
-
Reaction network of aldehyde hydrogenation over sulfided Ni?Mo/AlO catalysts作者:X WANG、R SALEH、U OZKANDOI:10.1016/j.jcat.2004.12.010日期:2005.4.1A reaction network of aldehyde hydrogenation over NiMoS/Al2O3 catalysts was studied with aldehydes with straight and branched carbon chains and different chain lengths as feed materials. The reactions in the gas phase and the liquid phase were compared. The main reaction in the aldehyde hydrogenation process is the hydrogenation of the CO double bond, which takes place over the coordinatively unsaturated以碳链为直链和支链,链长不同的醛为原料,研究了NiMoS / Al 2 O 3催化剂上醛加氢反应网络。比较了气相和液相中的反应。醛加氢过程中的主要反应是CO双键的加氢,该加氢发生在配位不饱和位点上。主要的副反应是醛的自缩合和醛与醇的缩合。这两个反应都涉及α-氢,并且主要由暴露的Al 2 O 3表面上的酸碱双功能位点催化。
-
Acetonyltriphenylphosphonium bromide in organic synthesis: a useful catalyst in the cyclotrimerization of aldehydes作者:Yung-Son Hon、Chia-Fu LeeDOI:10.1016/s0040-4020(01)00588-9日期:2001.7Acetonyltriphenylphosphonium bromide (ATPB) is a useful catalyst for the cyclotrimerization of the aliphatic aldehydes under solvent-free condition. The aldehydes tethered with a variety of functionality such as olefin, ether, ester, bromide, azide and diester could also be cyclotrimerized under the catalysis of ATPB.
-
Montmorillonite clay-catalyzed cyclotrimerization and oxidation of aliphatic aldehydes作者:Matthew R. Dintzner、Yawo A. Mondjinou、Dominic J. PileggiDOI:10.1016/j.tetlet.2009.12.009日期:2010.2A temperature-dependent equilibrium is observed for the cyclotrimerization of aliphatic aldehydes in the presence of Montmorillonite K10 clay, while aerobic oxidation of aliphatic aldehydes to the corresponding carboxylic acids is favored at room temperature in the presence of Montmorillonite KSF clay.
-
Novel Acidic Ionic Liquids as Efficient and Recyclable Catalysts for the Cyclotrimerization of Aldehydes作者:Heyuan Song、Jing Chen、Chungu Xia、Zhen LiDOI:10.1080/00397911.2010.523804日期:2012.1.15Abstract A mild, efficient, and ecofriendly procedure for cyclotrimerization of aldehydes was realized by using a series of novel Brønsted acidic ionic liquids (BAILs) consisting of double–SO3H groups in cations as catalysts. Good conversion of aldehydes and selectivity of trialkyl-1,3,5-trioxanes were achieved by using 1 mol% of BAILs. In addition, the catalyst system could be recycled and reused at
-
Allylstannation III. Synthesis of homoallylic alcohols and 4-chloro-2,6-dialkyl-3-methyltetrahydropyrans by reactions between (E/Z)-2-butenyldichloro-n-butyltin and aldehydes作者:Alessandro Gambaro、Andrea Boaretto、Daniele Marton、Giuseppe TagliaviniDOI:10.1016/0022-328x(83)80131-4日期:1983.10(in various cis/trans isomer ratios) reacts readily with neat RCHO (R = CH3, C2H5, (CH3)2CH, and C6H5) at 25°C to give (a) linear alcohols, RCH(OH)CH2CHCHCH3 in the E and Z forms, (b) branched alcohols, RCH(OH)CH(CH3)CHCH2 in the threo and erythro forms, and (c) 2,3,4,6-tetra-substituted tetrahydropyrans (A) as a mixture of cis/trans isomers arising from the CH(CH3)CHCl bond. The maximum yields25℃下,2-丁烯基二氯正丁基锡(以各种顺式/反式异构体比率)与纯净的RCHO(R = CH 3,C 2 H 5,(CH 3)2 CH和C 6 H 5)容易反应。得到的(a)的直链醇,RCH(OH)CH 2 CHCHCH 3中E和Z形式,(b)支链醇,RCH(OH)CH(CH 3)CHCH 2在苏式和赤式形式, (c)2,3,4,6-四取代的四氢吡喃(A),其为由CH(CH)产生的顺式/反式异构体的混合物3)CHCl键。在不存在溶剂的情况下,使用3-3.5摩尔比的RCHO /锡化合物可获得这些四氢吡喃的最大收率,而在CH 2 Cl 2中反应后的后处理则以线性醇为主要产物。线性醇的形成似乎是立体定向的,因为获得的E / Z异构体的比例与有机锡化合物中的比例相同。四氢吡喃优先形成为反式异构体。
表征谱图
-
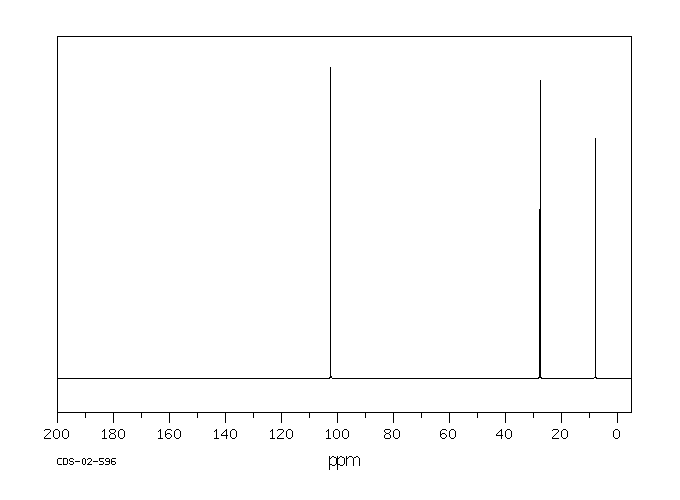
氢谱1HNMR
-
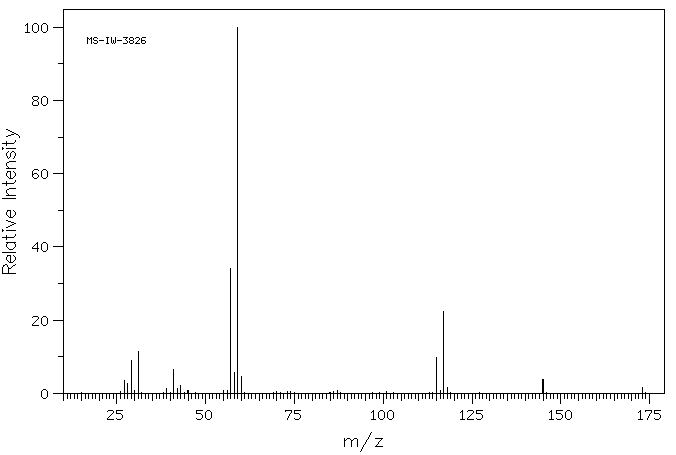
质谱MS
-
碳谱13CNMR
-
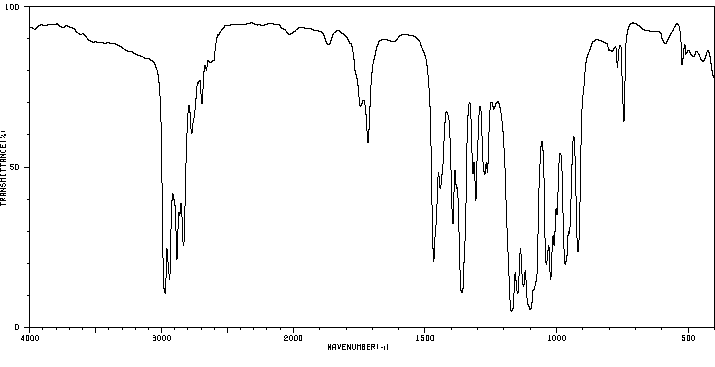
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
氯乙醛三聚物
三聚甲醛
三聚乙醛
S-三噁烷-13C3
9-氟-3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
6-甲基-2,4-二乙基-1,3,5-三恶烷
6,7a-二苯基螺[4a,7a-二氢-4aH-环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环己烷]-5-酮
4-(1,3,5-三氧杂环己烷-2-基)哒嗪
3-苯基-1,2,5-三氧杂螺[5.5]十一碳-7,10-二烯-9-酮
3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
2-氮杂二环[2.2.1]庚-5-烯-3-羧酸,乙基酯(9CI)
2-乙基-4,6-二甲基-1,3,5-三氧杂环己烷
2,4,6-三异丙基-1,3,5-三氧杂环己烷
2,4,6-三异丁基-1,3,5-三氧杂环己烷
2,4,6-三壬基-1,3,5-三氧杂环己烷
2,4,6-三乙基-1,3,5-三氧杂环己烷
2,4,6-三丙基-1,3,5-三氧杂环己烷
2,4,6-三(苯基甲基)-1,3,5-三氧杂环己烷
2,4,6-三(二氯甲基)-1,3,5-三氧杂环己烷
2,4,6-三(2-氯乙基)-1,3,5-三氧杂环己烷
1,3-二氧杂环庚烷与1,3,5-三氧杂环己烷的聚合物
1,3,5-三氧杂环己烷与环氧乙烷的聚合物
1,3,5-三氧杂环己烷与2,2-1,4-丁烷二基二(氧基亚甲基)二环氧乙烷和1,3-二氧戊环的聚合物
1,3,5-三氧杂环己烷与1,3-二氧戊环的聚合物
(4aS,7aS)-6,7a-二苯基螺[7,4a,7a-三氢环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环戊烷]
(Z)-N,N-diethyl-3-(6'-methylspiro[tricyclo[3.3.1.13,7]decane-2,3'-[1,2,4]trioxan]-6'-yl)acrylamide
4-(1,2,4-Trioxolan-3-yl)-2-butanon
6-tert-butyl-3-methyl-1,2,4-trioxan-5-one
2,4-Dibutyl-6-pentyl-1,3,5-trioxan
5-Adamantylen-1,3-dioxan
2,4,6,8-Tetraaethyl-1,3,5,7-tetraxan
1-[1,3]Dioxetan-2-ylmethyl-cyclohexanecarbonitrile
4r,5c-bis-chloromethyl-2ξ-methyl-[1,3]dioxolane
p-Dioxen-dioxetan
5-(3-[1,3]dioxolan-2-yl-propyl)-1-methyl-2-(2-methyl-[1,3]dioxolan-2-yl)-9-aza-bicyclo[3.3.1]nonane
(2-Isopropyl-[1,3]dioxan-5-yl)-dimethyl-sulfonium
2-(4-Methyl-pentyl)-[1,3]dioxetane
(Z)-N,N-diethyl-3-(8-methyl-6,7,10-trioxa-spiro[4.5]dec-8-yl)-acrylamide
β-Cyano-propionaldehyd-trimer, 2.4.6-Tris-2-cyano-aethyl-1.3.5-trioxan
(1,3,5-trioxane-2,4,6-triyl)trimethanamine
(3S,5R)-5-methoxy-3-methylspiro[1,2,4-trioxane-6,2'-adamantane]
12,12-Dimethyl-2,4,8,10-tetraoxa-tricyclo[4.4.4.01,6]tetradecane
1,6-Dichlor-1,6-dideoxy-2,4:3,5-di-O-methylen-L-idit
α-Multistriatin-d3
6-deoxy-2,4:3,5-di-O-methylene-L-gulitol
δ-Multistriatin-4,11,11-d3