2-甲氧基苯甲酸苯酯 | 10268-71-0
分子结构分类
中文名称
2-甲氧基苯甲酸苯酯
中文别名
——
英文名称
phenyl 2-methoxybenzoate
英文别名
2-methoxybenzoic acid phenyl ester;Phenyl-o-methoxybenzoat;2-Methoxybenzoesaeure-phenylester
CAS
10268-71-0
化学式
C14H12O3
mdl
MFCD00075799
分子量
228.247
InChiKey
LZRFQYZCMVMADF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:59 °C
-
沸点:369.0±25.0 °C(Predicted)
-
密度:1.159±0.06 g/cm3(Predicted)
-
保留指数:1859.4
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:应存放在室温、干燥且密封的环境中。
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:溶剂对取代苯甲酸苯基酯碱水解中邻位取代基作用的影响摘要:二阶速率常数ķ(DM 3 摩尔-1 小号-1为苯基酯的碱性水解)间位- ,对位和-邻-取代的苯甲酸在含水为5.3μm的NaClO 4和1.0M卜4测定NBR通过在25℃下的UV / Vis分光光度法。使用在各种介质中对苯甲酸的苯基酯进行碱水解的数据,研究了邻位感应,邻位共振以及间位和对极效应随溶剂参数的变化。的依赖邻上溶剂取代效果可通过下面的等式来精确描述的:Δlog ķ邻 =登录ķ邻 -日志ķ ħ = 0.059 + 2.19 σ我 + 0.304 σ ° - [R + 2.79 ë - 0.016Δ Eσ我 - 0.085Δ Eσ ° ř,其中ΔE是溶剂的亲电性,ΔE = E S - E H2O,表征了溶剂的氢键给体能力。在增加元和随着溶剂氢键供体容量(亲电子性)的降低,对极性取代基的影响与邻位取代基的共振项大致相同(-0.068ΔEσ ° m,p)。邻取代基的空间术语与DOI:10.1002/poc.1628
-
作为产物:描述:参考文献:名称:Titherley; Hughes, Journal of the Chemical Society, 1911, vol. 99, p. 1505摘要:DOI:
文献信息
-
Facile <i>N</i>-Arylation of Amines and Sulfonamides and <i>O</i>-Arylation of Phenols and Arenecarboxylic Acids作者:Zhijian Liu、Richard C. LarockDOI:10.1021/jo0602221日期:2006.4.1An efficient, transition-metal-free procedure for the N-arylation of amines, sulfonamides, and carbamates and O-arylation of phenols and carboxylic acids has been achieved by allowing these substrates to react with a variety of o-silylaryl triflates in the presence of CsF. Good to excellent yields of arylated products are obtained under very mild reaction conditions. This chemistry readily tolerates
-
Transition‐Metal‐Free DMAP‐Mediated Aromatic Esterification of Amides with Organoboronic Acids作者:Tao Wang、Yanqing Wang、Kai Xu、Yuheng Zhang、Jiarui Guo、Lantao LiuDOI:10.1002/ejoc.202100478日期:2021.6.14A new, transition-metal-free, effective method for aromatic esterification of amides with organoboronic acids has been developed, leading to a wide range of benzoate derivatives with yields ranging from moderate to good. The catalytic reaction shows broad substrate scope and excellent functional group tolerance.
-
Acylation of oxindoles using methyl/phenyl esters <i>via</i> the mixed Claisen condensation – an access to 3-alkylideneoxindoles作者:Ramdas Sreedharan、Purushothaman Rajeshwaran、Pradeep Kumar Reddy Panyam、Saurabh Yadav、C. M. Nagaraja、Thirumanavelan GandhiDOI:10.1039/d0ob00789g日期:——Predominantly, aggressive acid chlorides and stoichiometric coupling reagents are employed in the acylating process for synthesizing carbonyl tethered heterocycles. Herein, we report simple acyl sources, viz. methyl and phenyl esters, which acylate oxindoles via the mixed Claisen condensation. This straightforward protocol is mediated by LiHMDS and KOtBu and successfully applied to a wide range of
-
Palladium-Catalyzed Carbonylation of Aryl, Alkenyl, and Allyl Halides with Phenyl Formate作者:Tsuyoshi Ueda、Hideyuki Konishi、Kei ManabeDOI:10.1021/ol301192s日期:2012.6.15palladium-catalyzed carbonylation of aryl, alkenyl, and allyl halides with phenyl formate is reported. This procedure does not use carbon monoxide and affords one-carbon-elongated carboxylic acid phenyl esters in excellent yields. The reaction proceeds smoothly under mild conditions and tolerates a wide range of functional groups including aldehyde, ether, ketone, ester, and cyano groups. Furthermore, a variety
-
Palladacycle-Catalyzed Carbonylation of Aryl Iodides or Bromides with Aryl Formates作者:Guangwei Chen、Yuting Leng、Fan Yang、Shiwei Wang、Yangjie WuDOI:10.1002/cjoc.201300675日期:2013.12palladacycle‐catalyzed aromatic carbonylation reaction of aryl formates with aryl iodides or bromides has been developed. Commercially available and easily prepared aryl formates were employed as carbonyl sources without the use of external carbon monoxide. The present catalytic system shows broad functional group tolerance and affords aryl benzoate derivatives in good to excellent yields.
表征谱图
-
氢谱1HNMR
-
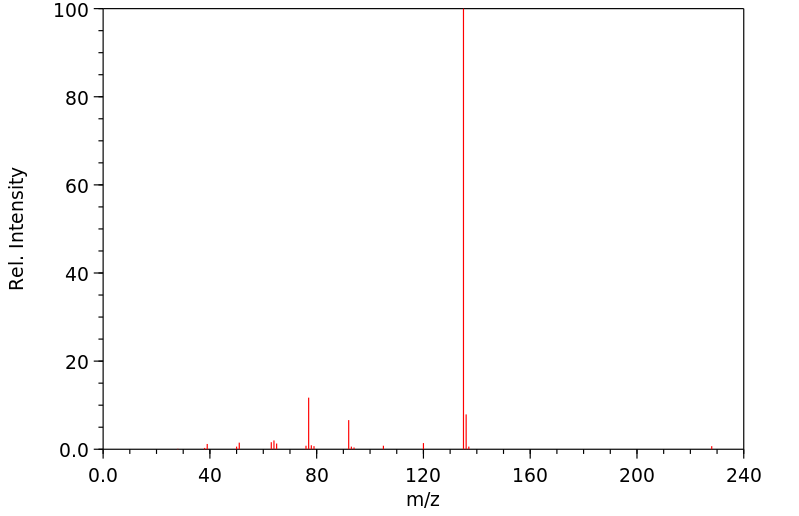
质谱MS
-
碳谱13CNMR
-
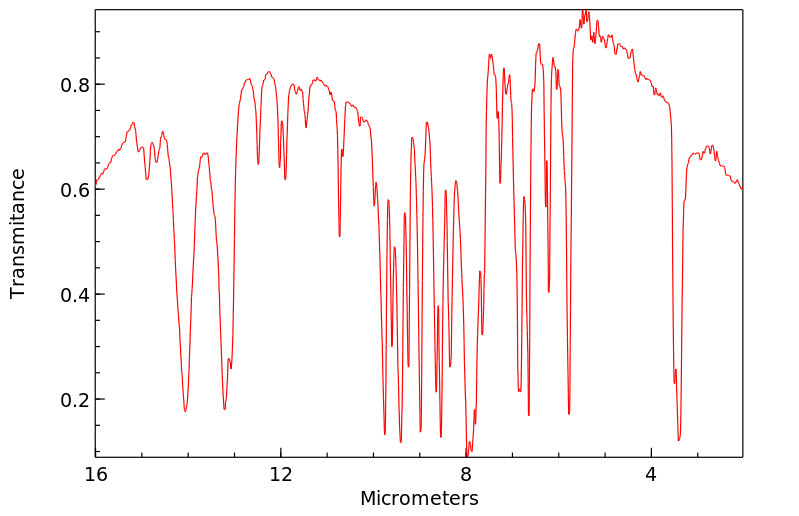
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
非那米柳
雷尼替丁
降钙素(humanreduced),8-L-缬氨酸-(9CI)
间苯甲酰氧基苯乙酮
间苯二甲酸二苯酯
间甲苯基苯甲酸酯
间双没食子酸
醋氨沙洛
邻苯二甲酸苄酯2-乙己基酯
邻苯二甲酸二苯酯-D4
邻苯二甲酸二苯酯
邻甲苯基苯甲酸酯
邻氨基苯甲酸(4-硝基苯基)酯
邻亚苯基二苯甲酸酯
贝诺酯
袋衣酸
血竭黄烷A
萘-1,5-二磺酸-4-[2-(二甲氨基)乙氧基]-2-甲基-5-(丙烷-2-基)苯基2-氨基苯酸酯(1:1)
茶痂衣酸
苯酚,2-[2-[(4-氯苯基)氨基]-4-噻唑基]-,苯酸酯(ester)
苯甲醯柳酸甲酯
苯甲酸苯酯
苯甲酸五氟苯酯
苯甲酸丁香酚酯
苯甲酸4-[[(4-甲氧基苯基)亚甲基]氨基]苯基酯
苯甲酸4-(乙酰氨基)-2-[[2-[4-(乙酰氨基)苯甲酰基]亚肼基]甲基]苯基酯
苯甲酸2-(2-苯并恶唑基)苯酯
苯甲酸-4-甲基苯酯
苯甲酸-(4-环戊基-苯基酯)
苯甲酸-(2-烯丙基-4-溴-苯基酯)
苯甲酸-(2-溴-4,6-二硝基-苯基酯)
苯甲酸-(2,4-二溴-3-甲基-苯基酯)
苯甲酸-(2,4-二氯-5-甲基-苯基酯)
苯甲酸-(2,4-二叔丁基苯基酯)
苯甲酸,4-羟基-,4-[(4-羟基苯氧基)羰基]苯基酯
苯甲酸,4-羟基-,4-(己氧基)苯基酯
苯甲酸,4-羟基-,4-(十四烷氧基)苯基酯
苯甲酸,4-羟基-,4-(十二烷氧基)苯基酯
苯甲酸,4-甲酰基-,4-(辛氧基)苯基酯
苯甲酸,4-甲氧基-,2-甲酰基苯基酯
苯甲酸,4-甲基-,4-甲基苯基酯
苯甲酸,4-戊基-,4-(壬氧基)苯基酯
苯甲酸,4-丁氧基-,1,4-亚苯基酯
苯甲酸,4-[[[3-[(2,2-二甲基-1-羰基丙氧基)甲基]-3,4-二氢-2-甲基-4-羰基-6-喹唑啉基]甲基]-2-炔丙基氨基]-,五氟苯基酯
苯甲酸,4-[1-(己氧基)乙基]-,4-(辛氧基)苯基酯
苯甲酸,4-(辛氧基)-,4-[[4-[[(1-甲基庚基)氧代]羰基]苯基]乙炔基]苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(癸氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(壬氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(十二烷氧基)苯基酯
苯甲酸,4-(癸氧基)-,4-[氰基[(1-羰基戊基)氧代]甲基]苯基酯,(R)-