三甲基-丙基锡烷 | 3531-45-1
分子结构分类
中文名称
三甲基-丙基锡烷
中文别名
——
英文名称
n-propyltrimethyltin
英文别名
Trimethyl-propyl-stannan;Trimethyl-propyl-zinn;n-Propyl-trimethyl-zinn;n-Propyl-trimethyl-stannan;Stannane, trimethylpropyl-;trimethyl(propyl)stannane
CAS
3531-45-1
化学式
C6H16Sn
mdl
——
分子量
206.903
InChiKey
GDLIQYVPJMAUEA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:799;799;801;833
计算性质
-
辛醇/水分配系数(LogP):2.73
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2931900090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:碳氢化合物-破伤风破灭的卤化物一。饱和碳的亲电性替代摘要:卤素与四烷基锡之间的反应受到溶剂的介电常数,极化率和亲核性的强烈影响。溶剂作用的这三个方面与锡利用其空的5 d轨道的能力有关:«Sn极化,通过金属的五元配位增强,决定了与其相连的烷基的反应性,适当的注意空间效应的引入。DOI:10.1016/s0022-328x(00)91016-7
-
作为产物:参考文献:名称:一些锡烷的蒸气压摘要:DOI:10.1021/j150310a006
文献信息
-
Photochemistry of ethyltrimethylstannane and n-propyltrimethylstannane at 185 nm作者:Peter Borrell、A. E. PlattDOI:10.1039/tf9706602279日期:——Ethyltrimethylstannane vapour (ets) yields several gaseous hydrocarbons on photolysis together with a yellow polymer which deposits on the cell window. Addition of nitrogen or argon (130 mbar) reduces the yields by 90 % but there is a residual reaction which is unaffected by pressure of inert gas. Addition of oxygen reduces some yields to zero. The major primary reactions are C2H5Sn(CH3)3+hν(λ= 185 nm)→C2H4+(CH3)3SnH, ϕ∼0.40; →C2H5+CH3+Sn(CH3)2, ϕ∼0.55. The quantum yields of formation of the radical products are increased by radical abstraction from the stannane. Similar results were found for propyltrimethylstannane (pts) but the principal free radical reaction is C3H7Sn(CH3)3+hν(λ= 185 nm)→ C3H7+ 2CH3+ Sn(CH3). The quenching effect is attributed to the deactivation of one of the two excited state precursors of the reaction.乙基三甲基锡烷蒸气(ets)在光解过程中生成几种气态烃以及沉积在反应室窗口上的黄色聚合物。加入氮气或氩气(130 毫巴)可将产率降低 90%,但存在一种不受惰性气体压力影响的残余反应。加入氧气可使某些产率降至零。主要初级反应为:C2H5Sn(CH3)3 + hν(λ= 185 nm) → C2H4 + ( )3SnH,量子产率 ϕ ≈ 0.40;→ C2H5 + + Sn( )2,量子产率 ϕ ≈ 0.55。锡烷中自由基的抽取使得自由基产物的形成量子产率增加。对于丙基三甲基锡烷(pts)也得到了类似结果,但其主要自由基反应为 Sn( )3 + hν(λ= 185 nm) → C3H7 + 2 + Sn( )。熄灭效应归因于反应的两个激发态前体之一的失活。
-
Organometallic compounds作者:M. Gielen、M.R. Barthels、M. De Clercq、C. Dehouck、G. MayenceDOI:10.1016/s0022-328x(00)88289-3日期:1972.1Diastereotopic nonequivalence is described for molecules of the type R′Me2-SnCHY(CH3) (with YC2H5, C6H5 and with various R′ groups), for meso-R″2 Sn(CHYCH3)2 (with R″CH3, CH2C6H5) and for diastereoisomers such as Me4-n- Sn(CHZCH3)n [ZEt, Pr; n2,3] and Me-i-Pr-cyclo-HexSnCH(CH3) (C6H5).非对映异构非对等是该类型R'Me的分子描述2 -SnCHY(CH 3)(与YC 2 ħ 5,C 6 H ^ 5并与各R'基团),对于内消旋-R“ 2 SN(CHYCH 3)2(具有R” = CH 3,CH 2 C 6 H 5),对于非对映异构体,例如Me 4- n - Sn(CHZCH 3)n [Z = Et,Pr; Ñ 2,3]和Me-I-PR-环HexSnCH(CH 3)(C 6 H ^ 5)。
-
Charge-transfer mechanism for electrophilic reactions. SE2 cleavage of alkylmetals with iodine作者:S. Fukuzumi、J. K. KochiDOI:10.1021/ja00527a001日期:1980.3
-
Donor-acceptor complexes of organometals and iodine. Alkyl ligands as probes for steric effects in charge transfer作者:S. Fukuzumi、J. K. KochiDOI:10.1021/j100443a009日期:1980.3
-
Formation constants of charge-transfer complexes. Steric effects in alkylmetal donors作者:S. Fukuzumi、J. K. KochiDOI:10.1021/j100443a010日期:1980.3
表征谱图
-
氢谱1HNMR
-
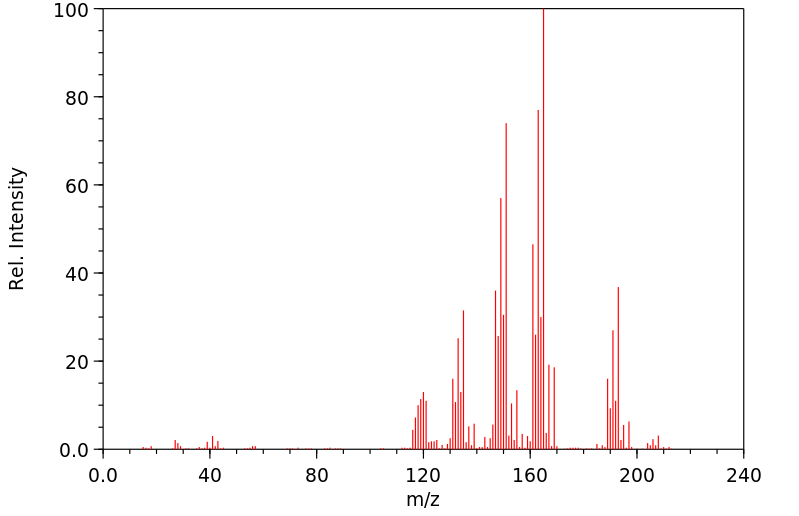
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝