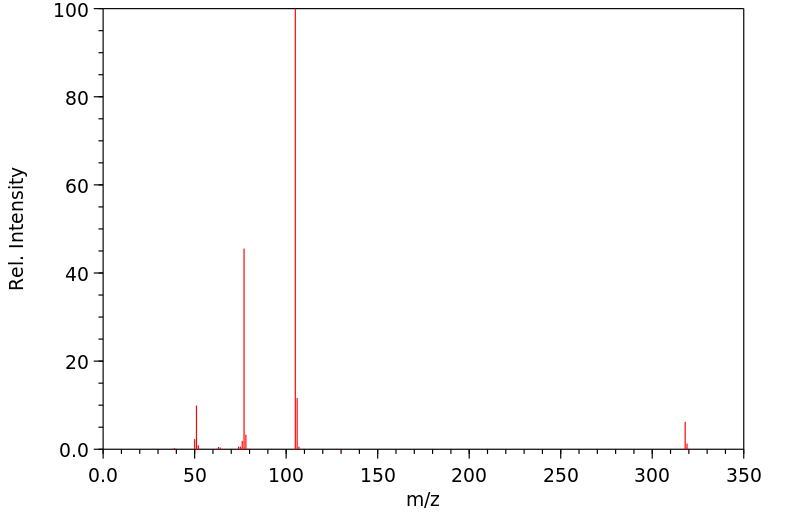
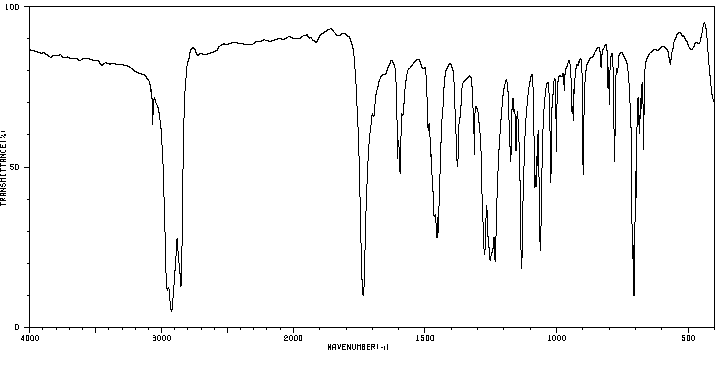
1,3-二苯甲酰氧基苯 | 94-01-9
中文名称
1,3-二苯甲酰氧基苯
中文别名
间亚苯基二苯甲酸酯;1,3-联苯甲酰氧基苯;1,3-苯二醇二苯甲酸酯;亚苯基间二苯甲酸酯;间苯二酚双苯甲酸酯;间苯二酚二苯甲酸酯
英文名称
resorcinol dibenzoate
英文别名
benzene-1,3-diyl dibenzoate;1,3-benzenediol dibenzoate;benzene-1,3-diyl benzoate;1,3-dibenzoyloxy benzene;1,3-dibenzoyloxybenzene;m-phenylene dibenzoate;(3-benzoyloxyphenyl) benzoate
CAS
94-01-9
化学式
C20H14O4
mdl
——
分子量
318.329
InChiKey
SUQGLJRNDJRARS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115-117 °C
-
沸点:450 °C
-
密度:1.2086 (rough estimate)
-
闪点:93°C
-
溶解度:溶于丙酮
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:24
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2916399090
-
RTECS号:VH0590000
-
储存条件:本品应密封保存。
SDS
| Name: | M-Phenylene Dibenzoate Material Safety Data Sheet |
| Synonym: | Dibenzoate Resourcino |
| CAS: | 94-01-9 |
Synonym:Dibenzoate Resourcino
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 94-01-9 | 1,3-Benzenediol, Dibenzoate | ca 100 | 202-294-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 94-01-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white to off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 157 deg C
Freezing/Melting Point: 114.00 - 118.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C20H14O4
Molecular Weight: 318.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 94-01-9: VH0590000 LD50/LC50:
Not available.
Carcinogenicity:
1,3-Benzenediol, Dibenzoate - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 94-01-9: No information available.
Canada
CAS# 94-01-9 is listed on Canada's NDSL List.
CAS# 94-01-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 94-01-9 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-苯二酚单苯甲酸酯 benzoate d'hydroxy-3 phenyle 136-36-7 C13H10O3 214.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3-苯二酚单苯甲酸酯 benzoate d'hydroxy-3 phenyle 136-36-7 C13H10O3 214.221 —— Benzoic acid 3-(10-acetoxymethyl-anthracen-9-ylmethoxy)-phenyl ester 885481-28-7 C31H24O5 476.529 —— 4-Benzoyloxy-2-hydroxy-benzophenon 18803-25-3 C20H14O4 318.329
反应信息
-
作为反应物:描述:参考文献:名称:在非水解条件下温和的化学选择性方法对乙酸芳基酯和苯甲酸酯进行脱保护摘要:乙酸芳基酯和苯甲酸酯的化学选择性脱保护可以在非水解条件下通过在100°C下于N-甲基-2-吡咯烷酮(NMP)中用K 2 CO 3处理来实现。在分子内竞争期间,在烷基羧酸盐的存在下,乙酸芳基酯和苯甲酸酯的选择性裂解发生。DOI:10.1016/s0040-4020(01)00922-x
-
作为产物:描述:参考文献:名称:三氟甲烷磺酸作为在用酸酐和混合酸酐酰化醇时极活泼的路易斯酸催化剂。摘要:可商购的三氟甲磺酸(三氟甲磺酸late)是一种实用且有用的路易斯酸催化剂,用于在对硝基苯甲酸酐的存在下将醇与酸酐酰化或将醇通过羧酸酯化。三氟甲磺酸scan的极高的催化活性可用于协助伯醇的酸酐酰化以及空间受阻的仲或叔醇的酸酐酰化。所提出的方法对于ω-羟基羧酸的选择性大内酯化特别有效。DOI:10.1021/jo952237x
文献信息
-
HF–Pyridine: A Versatile Promoter for Monoacylation/Sulfonylation of Phenolic Diols and for Direct Conversion of <i>t</i>-Butyldimethylsilyl Ethers to the Corresponding Acetates作者:Kyosuke Michigami、Kazuya Yoshimoto、Masahiko HayashiDOI:10.1246/cl.2012.138日期:2012.2.5Monoacylation and trifluoromethanesulfonylation of phenolic diols were achieved by the aid of HF–pyridine, whereas diacylation occurred with pyridine alone. Furthermore, HF–pyridine was found to promote the direct conversion of t-butyldimethylsilyl ethers to the corresponding acetates.
-
Electrostatic catalysis by ionic aggregates: scope and limitations of Mg(ClO4)2 as acylation catalyst作者:Asit K Chakraborti、Lalima Sharma、Rajesh Gulhane、ShivaniDOI:10.1016/j.tet.2003.08.007日期:2003.9Alkali and alkaline earth metal perchlorates exhibit electrostatic catalysis in the activation of anhydrides for the acylation reaction. Perchlorates with higher values of the charge-size function of the metal ion exhibit better catalytic activity following the order Mg(ClO4)2>Ba(ClO4)2>LiClO4. Acylation of structurally diverse phenols, thiols, alcohols, and amines have been carried out with stoichiometric
-
Bismuth(III) salts as convenient and efficient catalysts for the selective acetylation and benzoylation of alcohols and phenols作者:Iraj Mohammadpoor-Baltork、Hamid Aliyan、Ahmad Reza KhosropourDOI:10.1016/s0040-4020(01)00521-x日期:2001.7Efficient acetylation and benzoylation of alcohols and phenols with acetic and benzoic anhydrides have been carried out under catalysis of bismuth(III) salts including BiCl3, Bi(TFA)3 and Bi(OTf)3. Selective acetylation and benzoylation of alcohols in the presence of phenols is an additional advantage of this procedure.
-
Solid-Phase Benzoylation of Phenols and Alcohols in Microwave Reactor: An Ecofriendly Protocol作者:Suchandra Chakraborty、Ahana Saha、Kaushik Basu、Chandan SahaDOI:10.1080/00397911.2015.1078899日期:2015.10.18Abstract An efficient solid-phase benzoylation of phenols and alcohols was developed under microwave irradiation. A stoichiometric amount of benzoyl chloride was sufficient to carry out the reaction. This benzoylation features short reaction time, good yields, and easy workup procedures. Furthermore, the scope of the reaction was extended to prepare 3,5-dinitrobenzoyl derivatives of alcohols. GRAPHICAL
-
PhCOCl-Py/Basic Alumina as a Versatile Reagent for Benzoylation in Solvent-Free Conditions
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
非那米柳
雷尼替丁
降钙素(humanreduced),8-L-缬氨酸-(9CI)
间苯甲酰氧基苯乙酮
间苯二甲酸二苯酯
间甲苯基苯甲酸酯
间双没食子酸
醋氨沙洛
邻苯二甲酸苄酯2-乙己基酯
邻苯二甲酸二苯酯-D4
邻苯二甲酸二苯酯
邻甲苯基苯甲酸酯
邻氨基苯甲酸(4-硝基苯基)酯
邻亚苯基二苯甲酸酯
贝诺酯
袋衣酸
血竭黄烷A
萘-1,5-二磺酸-4-[2-(二甲氨基)乙氧基]-2-甲基-5-(丙烷-2-基)苯基2-氨基苯酸酯(1:1)
茶痂衣酸
苯酚,2-[2-[(4-氯苯基)氨基]-4-噻唑基]-,苯酸酯(ester)
苯甲醯柳酸甲酯
苯甲酸苯酯
苯甲酸五氟苯酯
苯甲酸丁香酚酯
苯甲酸4-[[(4-甲氧基苯基)亚甲基]氨基]苯基酯
苯甲酸4-(乙酰氨基)-2-[[2-[4-(乙酰氨基)苯甲酰基]亚肼基]甲基]苯基酯
苯甲酸2-(2-苯并恶唑基)苯酯
苯甲酸-4-甲基苯酯
苯甲酸-(4-环戊基-苯基酯)
苯甲酸-(2-烯丙基-4-溴-苯基酯)
苯甲酸-(2-溴-4,6-二硝基-苯基酯)
苯甲酸-(2,4-二溴-3-甲基-苯基酯)
苯甲酸-(2,4-二氯-5-甲基-苯基酯)
苯甲酸-(2,4-二叔丁基苯基酯)
苯甲酸,4-羟基-,4-[(4-羟基苯氧基)羰基]苯基酯
苯甲酸,4-羟基-,4-(己氧基)苯基酯
苯甲酸,4-羟基-,4-(十四烷氧基)苯基酯
苯甲酸,4-羟基-,4-(十二烷氧基)苯基酯
苯甲酸,4-甲酰基-,4-(辛氧基)苯基酯
苯甲酸,4-甲氧基-,2-甲酰基苯基酯
苯甲酸,4-甲基-,4-甲基苯基酯
苯甲酸,4-戊基-,4-(壬氧基)苯基酯
苯甲酸,4-丁氧基-,1,4-亚苯基酯
苯甲酸,4-[[[3-[(2,2-二甲基-1-羰基丙氧基)甲基]-3,4-二氢-2-甲基-4-羰基-6-喹唑啉基]甲基]-2-炔丙基氨基]-,五氟苯基酯
苯甲酸,4-[1-(己氧基)乙基]-,4-(辛氧基)苯基酯
苯甲酸,4-(辛氧基)-,4-[[4-[[(1-甲基庚基)氧代]羰基]苯基]乙炔基]苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(癸氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(壬氧基)苯基酯
苯甲酸,4-(苯基甲氧基)-,4-(十二烷氧基)苯基酯
苯甲酸,4-(癸氧基)-,4-[氰基[(1-羰基戊基)氧代]甲基]苯基酯,(R)-