2-硝基噻吩-4-腈 | 42137-23-5
中文名称
2-硝基噻吩-4-腈
中文别名
2-硝基噻吩-4-甲腈
英文名称
3-cyano-5-nitrothiophene
英文别名
5-nitro-thiophene-3-carbonitrile;2-Nitrothiophene-4-carbonitrile;5-nitrothiophene-3-carbonitrile
CAS
42137-23-5
化学式
C5H2N2O2S
mdl
——
分子量
154.149
InChiKey
NACMZLGGHMPMKQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:121-123°C
-
沸点:276.1±25.0 °C(Predicted)
-
密度:1.50±0.1 g/cm3(Predicted)
-
稳定性/保质期:
常规情况下不会分解,没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:97.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S22,S36/37
-
危险类别码:R20/21/22
-
海关编码:2934999090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:UN 3276
-
储存条件:密封、阴凉、干燥保存。
SDS
| Name: | 5-Nitrothiophene-3-carbonitrile 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 42137-23-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 42137-23-5 | 5-Nitrothiophene-3-carbonitrile | 97% | unlisted |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 42137-23-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 121 - 123 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H2N2O2S
Molecular Weight: 154
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Reducing agents, oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 42137-23-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Nitrothiophene-3-carbonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: NITRILES, SOLID, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
IMO
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
RID/ADR
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 42137-23-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 42137-23-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 42137-23-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— C-(5-Nitro-thiophen-3-yl)-methylamine 771582-78-6 C5H6N2O2S 158.181 5-硝基噻吩-3-羧酸 5-nitro-thiophene-3-carboxylic acid 40357-96-8 C5H3NO4S 173.149 —— 2-amino-4-cyanothiophene 159824-95-0 C5H4N2S 124.166
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of highly potent and selective hetaryl ureas as integrin αVβ3-Receptor antagonists摘要:Solid-phase synthesis and SAR of integrin alpha(v)beta(3)-receptor antagonists containing a urea moiety as non-basic guanidine mimetic are described. The most potent compounds exhibited IC50 values towards alpha(v)beta(3) in the nanomolar range and high selectivity versus related integrins like alpha(11b)beta(3). For selected examples efficacy in functional cellular assays is demonstrated. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(02)00161-0
文献信息
-
The ambident electrophilic behavior of 5-nitro-3-X-thiophenes in σ-complexation processes作者:Wahiba Gabsi、Khalid Essalah、Régis Goumont、Bahoueddine Tangour、Taoufik BoubakerDOI:10.1002/kin.21190日期:2018.9terms of Brønsted relationships reveals that the reaction mechanism likely involves a single‐electron transfer (SET) process. The excellent correlations upon plotting the rate constants versus the oxidation potentials Eo values is an additional evidence that reactions between thiophenes and phenoxide anions are proceeding through an initial electron transfer. It is of particular interest to note that the在20°C的水溶液中研究了3,5-二硝基噻吩1和3-氰基-5-硝基噻吩2与一系列对位取代的酚盐阴离子3a–c的反应动力学。已确定两个噻吩的两个未取代的亲电子中心(C(2)和C(4))。Fukui函数正确地将C(2)和C(4)原子预测为这些电子不足的噻吩1和2的最亲电子中心。根据布朗斯台德关系对实验数据进行分析表明,反应机理可能涉及单电子转移(SET)过程。绘制速率常数与氧化电位E o时的极好的相关性值是噻吩和酚盐阴离子之间的反应正在通过初始电子转移进行的另一个证据。特别值得注意的是,本文研究的系统提供了σ络合反应中SET机理的罕见例子。根据自由能关系日志ķ =小号(Ñ + ë)(Angew化学杂志,诠释。编英格兰,1994,33,938-957),亲电性参数È已经确定了噻吩的C-4和C-2位置,并将其与其他环境亲电试剂的反应性进行了比较。另一方面,这些噻吩与氢氧根离子反应的二阶速率常数已在
-
Discovery of a Novel, Selective, and Orally Bioavailable Class of Thrombin Inhibitors Incorporating Aminopyridyl Moieties at the P1 Position作者:Dong-Mei Feng、Stephen J. Gardell、S. Dale Lewis、Mark G. Bock、Zhongguo Chen、Roger M. Freidinger、Adel M. Naylor-Olsen、Harri G. Ramjit、Richard Woltmann、Elizabeth P. Baskin、Joseph J. Lynch、Robert Lucas、Jules A. Shafer、Kimberley B. Dancheck、I-Wu Chen、Shi-Shan Mao、Julie A. Krueger、Timothy R. Hare、Anne M. Mulichak、Joseph P. VaccaDOI:10.1021/jm970493r日期:1997.11.1A novel class of thrombin inhibitors incorporating aminopyridyl moieties at the P1 position has been discovered. Four of these thrombin inhibitors (13b,c,e and 14d) showed nanomolar potency (K-i 0.8-12 nM), 300-1500-fold selectivity for thrombin compared with trypsin, and good oral bioavailability (F = 40-76%) in rats or dogs. The neutral Fl was expected to increase metabolic stability and oral absorption. Identification of this novel aminopyridyl group at Fl was a key step in our search for a clinical candidate.
-
Yoshiizumi, Kazuya; Ikeda, Shoji; Nishimura, Noriyasu, Chemical and Pharmaceutical Bulletin, 1997, vol. 45, # 12, p. 2005 - 2010作者:Yoshiizumi, Kazuya、Ikeda, Shoji、Nishimura, Noriyasu、Yoshino, KohichiroDOI:——日期:——
-
Dell'Erba, Carlo; Sancassan, Fernando; Novi, Marino, Journal of the Chemical Society. Perkin transactions II, 1991, # 10, p. 1631 - 1636作者:Dell'Erba, Carlo、Sancassan, Fernando、Novi, Marino、Spinelli, Domenico、Consiglio, GiovanniDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
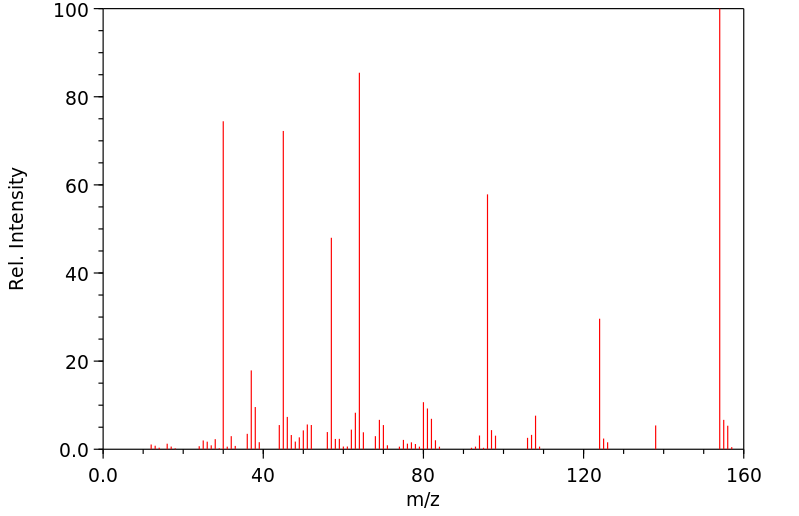
质谱MS
-
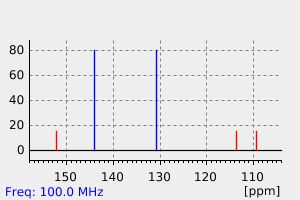
碳谱13CNMR
-
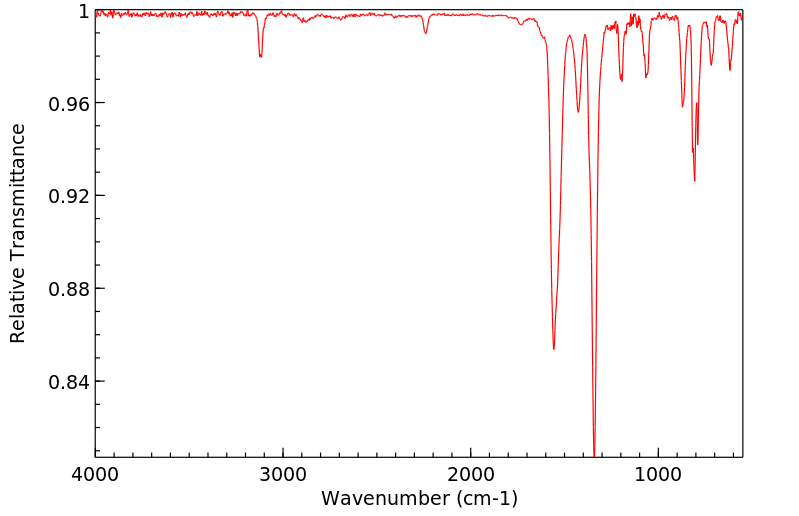
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯