2,2'-thenoyl disulfide | 116401-30-0
中文名称
——
中文别名
——
英文名称
2,2'-thenoyl disulfide
英文别名
bis-(thiophene-2-carbonyl)-disulfane;Bis-(thiophen-2-carbonyl)-disulfan;Disulfide, bis(2-thenoyl)-;S-(thiophene-2-carbonylsulfanyl) thiophene-2-carbothioate
CAS
116401-30-0
化学式
C10H6O2S4
mdl
——
分子量
286.42
InChiKey
ZPWFFRLQFIXCDO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:16
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:141
-
氢给体数:0
-
氢受体数:6
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-噻吩硫代甲S-酸 thiophene-2-monocarboxylic acid 49628-34-4 C5H4OS2 144.218
反应信息
-
作为产物:描述:参考文献:名称:Silver(I) catalyzed oxidation of thiocarboxylic acids into the corresponding disulfides and synthesis of some new Ag(I) complexes of thiophene-2-thiocarboxylate摘要:Aromatic thiocarboxylic acids in presence of a base on treatment with silver nitrate under ambient conditions were oxidized to the corresponding disulfides. The reactions were found to be catalyzed by Ag+ ions. The catalytic oxidation is paralleled by the Ag(SCOAr) complex formation reaction which could be considerably subsided by adjustment of the reaction conditions. Attempts to use (Ag(PPh3)(2)](+) or [Ag(PPh3)](+)r ion as the catalyst were unsuccessful as these resulted in the formation of the corresponding thiocarboxylate complexes. The products, ArCOSSCOAr (1, 2), [Ag(SCOAr)(PPh3)(2)] (3, 4) and [Ag(SCOAr)(PFh(3))](4) (5) (Ar = C6H5, C4H3S) were characterized by single crystal X-ray analysis. Compounds 3 and 4 are monomeric while 5 is a cyclic tetramer in the crystalline phase. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.poly.2010.09.033
文献信息
-
Bory et al., Annales Pharmaceutiques Francaises, 1954, vol. 12, p. 673作者:Bory et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
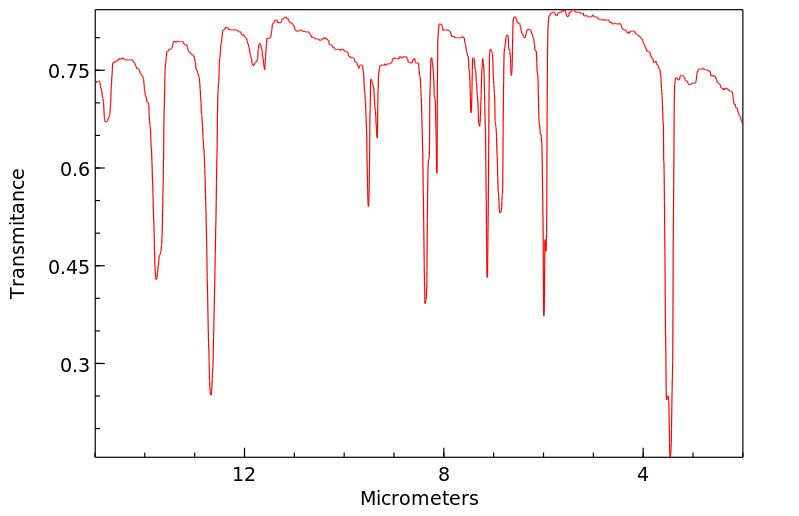
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯