[2,5-二(三甲基硅烷基)苯基]-三甲基硅烷 | 17864-15-2
中文名称
[2,5-二(三甲基硅烷基)苯基]-三甲基硅烷
中文别名
——
英文名称
1,2,4-tris(trimethylsilyl)benzene
英文别名
1,2,4-Tris-trimethylsilyl-benzol;Benzene, 1,2,4-tris-trimethylsilyl-;[2,4-bis(trimethylsilyl)phenyl]-trimethylsilane
CAS
17864-15-2
化学式
C15H30Si3
mdl
——
分子量
294.66
InChiKey
JBUNNBYNEZNCGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.32
-
重原子数:18
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2931900090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Felix,G. et al., Angewandte Chemie, 1977, vol. 89, p. 502 - 504摘要:DOI:
-
作为产物:描述:三甲基乙炔基硅 在 9,10-二联苯蒽 、 N,N-二异丙基乙胺 、 iron(II) chloride 作用下, 以 乙腈 为溶剂, 反应 1.0h, 以80%的产率得到[2,5-二(三甲基硅烷基)苯基]-三甲基硅烷参考文献:名称:联合光氧化还原和铁催化炔烃的环三聚。摘要:可见光光催化与金属催化的成功结合最近使得迄今为止未知的化学反应得以发展。将无金属光催化剂和地球丰富的金属催化剂合并的双重机制仍处于起步阶段。我们报道了通过无配体铁催化剂的光氧化还原活化来进行光有机铁催化的炔烃环三聚反应。该反应在非常温和的条件下进行(可见光,20 °C,1 小时),三种催化剂(染料、胺、FeCl 2)的负载量为 1–2 mol%。DOI:10.1002/anie.202000907
文献信息
-
A simple cobalt catalyst system for the efficient and regioselective cyclotrimerisation of alkynes作者:Gerhard Hilt、Thomas Vogler、Wilfried Hess、Fabrizio GalbiatiDOI:10.1039/b417832g日期:——The intermolecular cyclotrimerisation of terminal and internal alkynes can be catalysed by simple cobalt complexes such as a CoBr2(diimine) under mild reaction conditions when treated with zinc and zinc iodide with high regioselectivity in excellent yields.
-
Reduction of Molybdenum(V) Chloride with Various Reducing Metals: Reactivity Correlations with the Descendant Lewis Acids作者:Ryuichiro Hara、Qiaoxia Guo、Tamotsu TakahashiDOI:10.1246/cl.2000.140日期:2000.2Reactivity of low-valent molybdenum prepared from MoCl5 with various reducing metals in DME, was dependent on the reducing metals in the order of Al > Sn, In > Zn, Mg, Li in the case of cyclotrimerization of alkynes. This order is parallel to the acidity of the descendant Lewis acids.
-
Highly Regioselective Alkyne Cyclotrimerization Catalyzed by Titanium Complexes Supported by Proximally Bridged <i>p-tert-</i>Butylcalix[4]arene Ligands作者:Oleg V. Ozerov、Folami T. Ladipo、Brian O. PatrickDOI:10.1021/ja990740b日期:1999.9.1assembling complex organic molecules.1 Many transition metals catalyze the cyclotrimerization of alkynes to yield substituted benzenes.1,2 However, the reaction rarely proceeds with high regioselectivity.1d Highly regiocontrolled synthesis of arenes is very attractive since arenes are important building blocks in organic synthesis. Our interest in the influence of ancillary ligands on organic transformations许多用于组装复杂有机分子的最有用的合成方法都涉及过渡金属催化的环加成反应。1 许多过渡金属催化炔烃的环三聚反应生成取代苯。 1,2 然而,该反应很少以高区域选择性进行。1d 高度芳烃的区域控制合成非常有吸引力,因为芳烃是有机合成中的重要组成部分。我们对辅助配体对第 4 族金属介导的有机转化的影响的兴趣使我们合成了由 1,2-交替、二甲基甲硅烷基桥连的对叔丁基杯 [4] 芳烃 (DMSC) 配体支持的钛配合物。而醇盐已被有效地用作早期过渡金属有机金属化学中的辅助配体,3 杯芳烃作为辅助配体在有机过渡金属化学中的应用相对尚未探索。4 在本文中,我们描述了基于 DMSC 的钛配合物对末端炔烃的高区域选择性催化环三聚反应。(DMSC)H2 与 TiCl4 的反应以优异的收率提供 [(DMSC)TiCl2] (1),作为空气和水分敏感的橙色固体 6(方程式 1)。1 的 X 射线晶体学研究
-
A Mechanistic Study of the Utilization of <i>arachno</i> ‐Diruthenaborane [(Cp*RuCO) <sub>2</sub> B <sub>2</sub> H <sub>6</sub> ] as an Active Alkyne‐Cyclotrimerization Catalyst作者:K. Geetharani、Samat Tussupbayev、Julia Borowka、Max C. Holthausen、Sundargopal GhoshDOI:10.1002/chem.201200291日期:2012.7.2conditions yields the new metallaborane arachno‐[(Cp*RuCO)2B2H6] (2). Compound 2 catalyzes the cyclotrimerization of a variety of internal‐ and terminal alkynes to yield mixtures of 1,3,5‐ and 1,2,4‐substituted benzenes. The reactivities of nido‐1 a and arachno‐2 with alkynes demonstrates that a change in geometry from nido to arachno drives a change in the reaction from alkyne‐insertion to catalytic的反应巢- [1,2-(CP *期RuH)2乙3 ħ 7 ](1一个中,CP * =η 5 -C 5我5)与[沫(CO)3(CH 3 CN)3 ]在温和条件下产生新的金属laborane arachno -[(CP * RuCO)2 B 2 H 6 ](2)。化合物2催化多种内部和末端炔烃的环三聚反应,生成1,3,5-和1,2,4-取代的苯的混合物。Nido - 1a和Nido的反应性带有炔烃的arachno - 2证明,几何形状从Nido变为Arachno分别驱动了从炔烃插入到催化环三聚反应的变化。密度泛函计算已用于评估化合物2催化的炔烃环三聚反应的反应途径。该反应涉及钌环中间体的形成,随后的炔烃插入步骤是由该中间体与炔烃之间的[2 + 2]环加成反应引发的。实验和量子化学结果还表明,金属环中间体的稳定性在很大程度上取决于炔烃上存在的取代基的性质。
-
Iron-catalyzed regioselective cyclotrimerization of alkynes to benzenes作者:Suhas Shahaji Gawali、Chidambaram GunanathanDOI:10.1016/j.jorganchem.2018.12.007日期:2019.2catalyzed the regioselective [2+2+2] cyclotrimerization of terminal aryl and alkyl alkynes to provide the 1,2,4-trisubstituted benzene molecules. Interestingly, internal alkynes also exhibited similar cyclization and resulted in hexa-substituted benzene compounds. Increased steric bulk on pincer ligands diminished the selectivity for cycloaddition. Cyclotrimerization reactions proceeded at room temperature
表征谱图
-
氢谱1HNMR
-
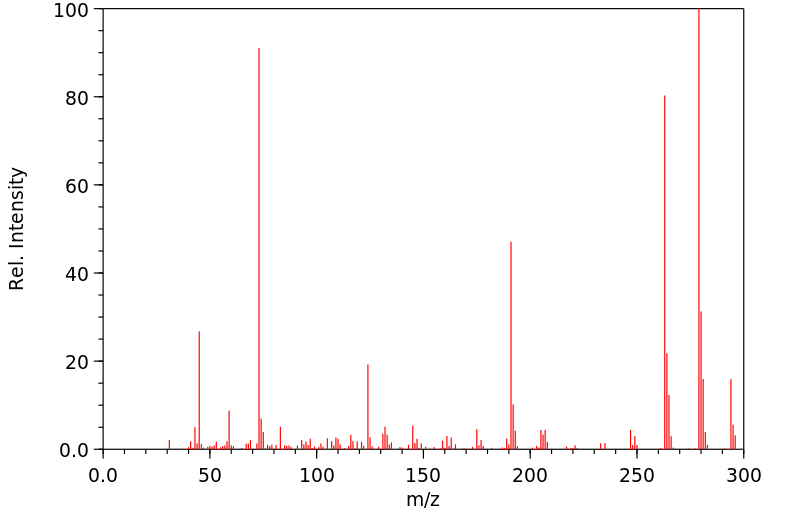
质谱MS
-
碳谱13CNMR
-
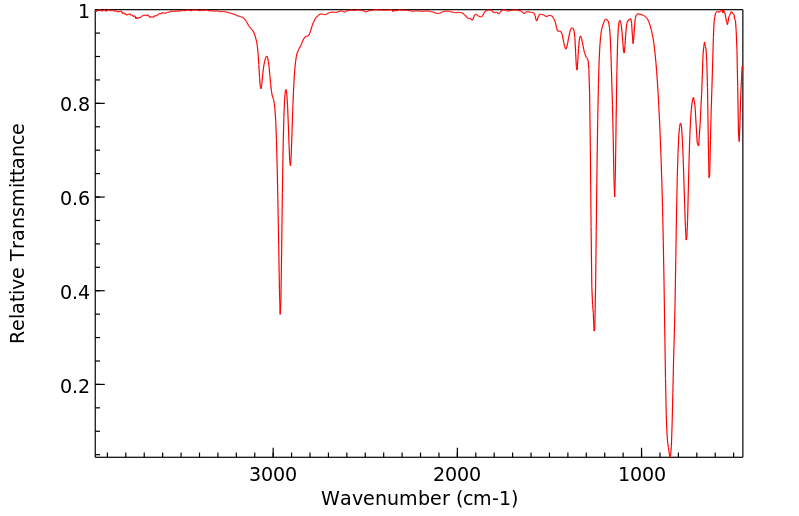
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷