4-甲基吗啉1,1-二氧化物 | 25343-91-3
中文名称
4-甲基吗啉1,1-二氧化物
中文别名
4-甲基硫代吗啉1,1-二氧化物
英文名称
N-methylthiomorpholine 1,1-dioxide
英文别名
4-methyl-thiomorpholine 1,1-dioxide;4-Methyl-thiomorpholin-1,1-dioxid;4-methyl thiomorpholine 1,1-dioxide;4-methylthiomorpholine-1,1-dioxide;N-Methyl-thiomorpholin-1,1-dioxyd;4-Methylthiomorpholin-1,1-dioxid;4-Methylthiomorpholine 1,1-dioxide;4-methyl-1,4-thiazinane 1,1-dioxide
CAS
25343-91-3
化学式
C5H11NO2S
mdl
MFCD00014623
分子量
149.214
InChiKey
ULZCOWMSBOJCLT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:84-88°C
-
沸点:148 °C(Press: 6 Torr)
-
密度:1.202±0.06 g/cm3(Predicted)
-
稳定性/保质期:
避免接触强氧化物。
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
安全说明:S26,S36
-
储存条件:将物品存放在密封的容器中,并储存在阴凉、干燥的地方。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 硫代吗啉-1,1-二氧化物 thiomorpholine 1,1-dioxide 39093-93-1 C4H9NO2S 135.187 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 硫代吗啉-4-甲酰氯-1,1-二氧化物 thiomorpholin-4-carbonyl chloride 1,1-dioxide 39093-77-1 C5H8ClNO3S 197.642
反应信息
-
作为反应物:描述:参考文献:名称:Bock; Haberkorn; Herlinger, Arzneimittel-Forschung/Drug Research, 1972, vol. 22, # 9, p. 1564 - 1569摘要:DOI:
-
作为产物:描述:参考文献:名称:布朗斯台德酸离子液体作为有机反应的溶剂守恒催化剂摘要:制备了含磺酰基的铵基布朗斯台德酸离子液体,并将其用作有机反应的液体多相催化剂。IL在反应体系中独特的宏观相异质性不仅确保了IL催化剂的出色催化活性,而且避免了使用有机反应溶剂。催化剂体系适用于多种反应。DOI:10.1002/cssc.201402220
文献信息
-
Variation of the ease of α-sulfonyl carbanion formation with the orientation of different β-substituents: Experimental evidence for the generality of negative hyperconjugation as an important substituent effect作者:James F King、Manqing Li、Allan Zijun Cheng、Vinod Dave、Nicholas C PayneDOI:10.1139/v03-015日期:2003.6.1Following up on our previous observation that the rate of formation of a β-alkoxy-substituted α-sulfonyl carbanion depends on the stereochemistry of the alkoxy group, we have found similar behaviour when the β-substituent is R2N, RS, or R3N+. With each substituent, the variation of kN (defined by kN = (kexch)X /(kexch)model) is consistent with an equation of the form log kN = a + b cos2θ, where θ is
-
Quinoline and quinazoline compounds useful in therapy申请人:——公开号:US20030045525A1公开(公告)日:2003-03-06Compounds of formula I, 1 wherein R 1 represents C 1-4 alkoxy optionally substituted by one or more fluorine atoms; R 2 represents an aryl group or a heteroaryl group, optionally substituted by C 1-4 alkyl or SO 2 NH 2 ; R 3 represents a 4-, 5-, 6-, or 7-membered heterocyclic ring containing at least one heteroatom selected from N, O and S, the ring being optionally fused to a benzene ring or a 5- or 6-membered heterocyclic ring containing at least one heteroatom selected from N, O and S, the ring system as a whole being optionally substituted; X represents CH or N; and L is absent, or represents a cyclic group of formula Ia, 2 or represents a chain of formula Ib, 3 and pharmaceutically acceptable salts thereof, are useful in the treatment of a variety of disorders including benign prostatic hyperplasia.式I的化合物,其中R1代表C1-4烷氧基,可选地取代一个或多个氟原子;R2代表芳基或杂芳基,可选地取代为C1-4烷基或SO2NH2;R3代表含有N、O和S中至少一个杂原子的4、5、6或7成员杂环,该环可选地融合到苯环或含有N、O和S中至少一个杂原子的5或6成员杂环中,整体环系统可选地取代;X代表CH或N;L不存在,或代表式Ia的环状基团,或代表式Ib的链状基团,以及其药学上可接受的盐,可用于治疗包括良性前列腺增生在内的各种疾病。
-
Ionic Liquid‐Catalyzed C−C Bond Formation for the Synthesis of Polysubstituted Olefins作者:Haotian Lv、Feng Han、Ning Wang、Nan Lu、Zenghong Song、Jian Zhang、Chengxia MiaoDOI:10.1002/ejoc.202201222日期:2022.12.6Acidic ionic liquid was used as a metal-free and recyclable catalyst for the synthesis of polysubstituted olefins by activating of ethers in dimethyl carbonate as a green solvent. Density functional theory (DFT) calculations showed a hydrogen bonding effect during the reaction.
-
512. Divinyl sulphone and allied compounds作者:A. H. Ford-MooreDOI:10.1039/jr9490002433日期:——
-
Lawson; Reid, Journal of the American Chemical Society, 1925, vol. 47, p. 2829作者:Lawson、ReidDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
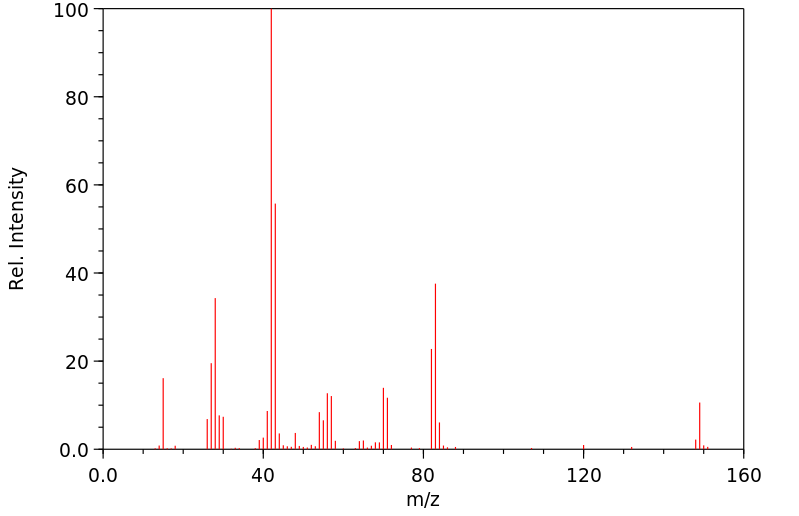
质谱MS
-
碳谱13CNMR
-
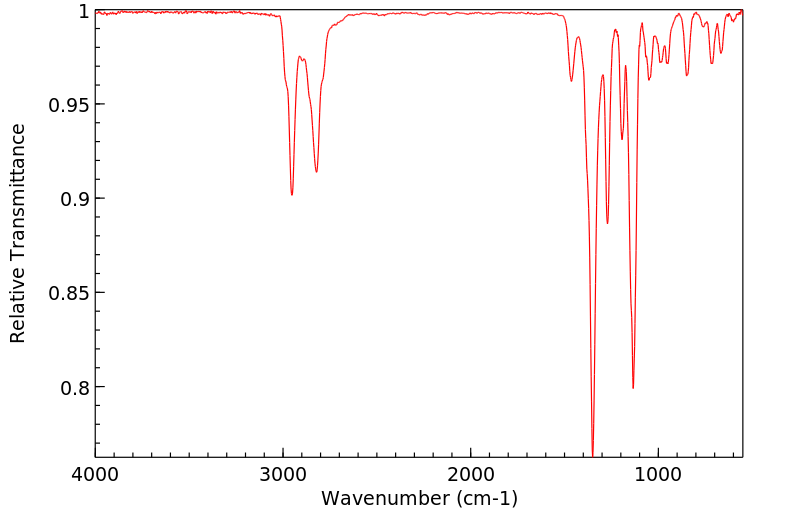
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3S,5S,6S)-安非他酮杂质
(3S,5R,6R)-安非他酮杂质
苯西酮
苯甲酸,4-(4-硫代吗啉基)-
硫代吗啉酮
硫代吗啉盐酸盐
硫代吗啉-4-醇1,1-二氧化物
硫代吗啉-4-甲酰氯-1,1-二氧化物
硫代吗啉-3-基甲醇
硫代吗啉-2-甲酸乙酯
硫代吗啉-1-鎓-1-醇
硫代吗啉-1,1-二氧化物
硫代吗啉,4-[4-[[2-(2,4-二氯苯基)-2-(1H-咪唑-1-基甲基)-1,3-二噁戊环-4-基]甲氧基]苯基]-,1-氧化,顺-(9CI)
硫代吗啉,3-乙基-2-甲基-
硫代吗啉 1,1-二氧化物盐酸盐
硫代吗啉
甲基2-乙氧基-6H-1,3-噻嗪-5-羧酸酯
甲基2-(甲基氨基)-4-氧代-5,6-二氢-4H-1,3-噻嗪-6-羧酸酯
甲基(2Z)-3-苄基-2-(苄基亚氨基)-4-氧代-1,3-噻嗪烷-6-羧酸酯
甲基(2Z)-3-异丙基-2-(异丙基亚胺)-4-氧代-1,3-噻嗪烷-6-羧酸酯
巯基吗啉-4-甲酸叔丁酯
四氢-3-甲基-2-苯基-4H-1,3-噻嗪-4-酮1,1-二氧化物
四氢-1,4-噻嗪-3,5-二酮
噻唑并[2,3-c][1,4]噻嗪-3(2H)-硫酮,8a-乙基四氢-8-甲基-
反式环戊烯三硫代碳酸酯
二苯甲基{5-[(4,6-二脱氧六吡喃糖基)氧代]-2,4,6-三羟基环己烷-1,3-二基}二(甲基氨基甲酸酯)
n-boc-2-硫代吗啉羧酸乙酯
[(2Z)-3-氰基-1,3-噻唑烷-2-亚基]氰胺
N-甲基四氢-1,2-噻嗪S,S-二氧化物
N-甲基-4-硫代吗啉甲酰胺
N-环己基-5,6-二氢-4H-1,3-噻嗪-2-胺
N-亚硝基硫代吗啉
N-丁基-5,6-二氢-4H-1,3-噻嗪-2-胺
N-Boc-1,4-噻嗪S,S-二氧化物
N-(3-氨基丙基)-硫代吗啉
N-(2-羟基丙基)硫代吗啉
N-(2-羟乙基)吗啉
AMT盐酸盐
6-苄基-2-甲基噻嗪1,1-二氧化物
6-羟基-5,6-二甲基-1,3-噻吖己环-2-硫酮
6-甲基-4-苯基硫代吗啉-3-酮
6-甲基-2-苯基-5,6-二氢-4H-1,3-噻嗪
6-甲基-1,3-噻嗪-2-硫酮
6-异丙基-3-硫代吗啉酮1-氧化物
6-异丙基-3-硫代吗啉酮
6-丙基-硫代吗啉-3-酮
6-(丁氧基甲基)-4-苯基硫代吗啉-3-酮
6,6-二甲基-1,4-噻嗪-2,5-二甲酸
5H-[1,3]噻唑并[5,4-h][1,4]苯并噻嗪
5-甲基-6-(吡啶-3-基)硫代吗啉-3-酮盐酸(1:1)