methylthio-trimethyl-tin | 993-46-4
分子结构分类
中文名称
——
中文别名
——
英文名称
methylthio-trimethyl-tin
英文别名
(Trimethyl)(methylthio)stannan;Methyl-trimethylstannyl-sulfid;Methylthio-trimethyl-stannan;Trimethyl(methylthio)stannan;Trimethyl-methylmercaptozinn;Trimethylzinnmethylmercaptid;Methanethiolate;trimethylstannanylium
CAS
993-46-4
化学式
C4H12SSn
mdl
——
分子量
210.915
InChiKey
KCZRVNHUBBEAPA-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:163 °C
计算性质
-
辛醇/水分配系数(LogP):2.18
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Ehrl, W.; Vahrenkamp, H., Chemische Berichte, 1970, vol. 103, p. 3563 - 3579摘要:DOI:
-
作为产物:描述:参考文献:名称:Dehnert, Peter; Grobe, Joseph; Van, Duc Le, Zeitschrift fur Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1981, vol. 36, # 1, p. 48 - 54摘要:DOI:
文献信息
-
Reactions of Group IV organometallic compounds. Part XI. Some insertion reactions of alkylsilyl and alkylstannyl sulphides作者:Kenji Itoh、Kimishige Matsuzaki、Yoshio IshiiDOI:10.1039/j39680002709日期:——Reaction of trimethylsilyl sulphide with phenyl isocyanate gives a 1 : 1 insertion product, but subsequent insertion of phenyl isocyanate takes place readily in the case of trialkylstannyl sulphide, to produce triphenyl isocyanurate. When chloral is used as a reactant for trialkyltin sulphides, substitution of all three chlorine atoms occurs, to afford trialkyltin chloride and (RS)3C·CHO, although
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: F: PerFHalOrg.9, 7, page 50 - 101作者:DOI:——日期:——
-
Aber, F. W.; Crow, J. P.; Wingfield, J. N., Journal of the Chemical Society, Dalton Transactions作者:Aber, F. W.、Crow, J. P.、Wingfield, J. N.DOI:——日期:——
-
Grobe, Joseph; Le Van, Duc, Zeitschrift fur Naturforschung, Teil B: Anorganische Chemie, Organische Chemie作者:Grobe, Joseph、Le Van, DucDOI:——日期:——
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Cr: Org.Verb., 1.1.2.1.4.1, page 41 - 54作者:DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
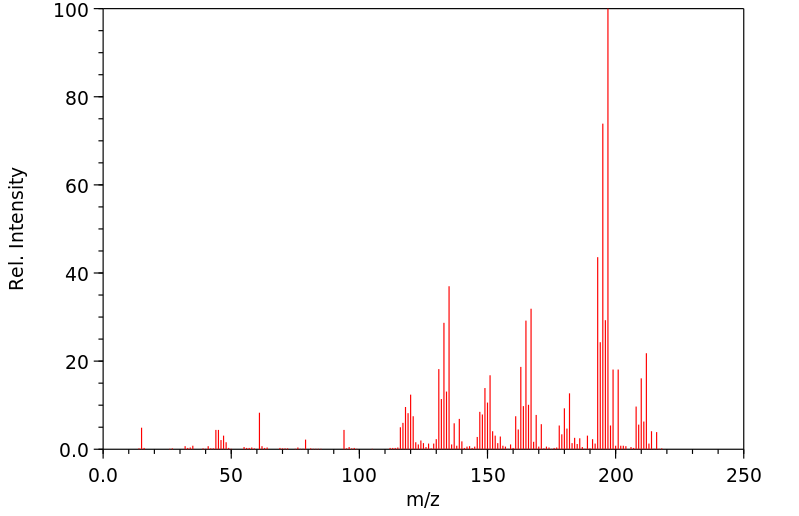
质谱MS
-
碳谱13CNMR
-
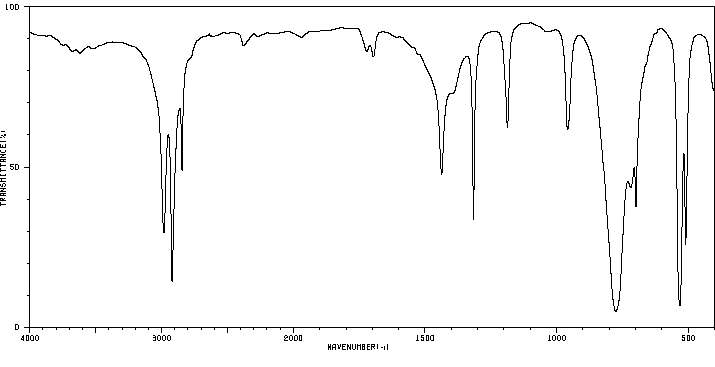
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝