ethyl 2-oxo-2H-cyclohepta[b]furan-3-carboxylate | 22442-46-2
中文名称
——
中文别名
——
英文名称
ethyl 2-oxo-2H-cyclohepta[b]furan-3-carboxylate
英文别名
3-ethoxycarbonyl-2H-cyclohepta[b]furan-2-one;Ethyl 2-oxo-2H-cyclohepta(b)furan-3-carboxylate;ethyl 2-oxocyclohepta[b]furan-3-carboxylate
CAS
22442-46-2
化学式
C12H10O4
mdl
——
分子量
218.209
InChiKey
JULPYSSLWYDUIT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:479.3±28.0 °C(Predicted)
-
密度:1.29±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Formation and structure of 2-diazo-2,4-azulenequinone derivatives摘要:The diazotization of methyl (and ethyl) 2-amino-3-cyano-4-methoxy(and ethoxy)azulene-1-carboxylate (1-4) was examined to determine whether the products are 2-diazo-2,4-azulenequinone derivatives (B) or azulene-2-diazonium-1-carboxylate derivatives (C). Since diazotization of 1 and 2 gave the same diazo compound 5 and diazotization of both 3 and 4-gave diazo compound 6, the diazo compounds are deduced to not be azulene-2-diazonium-1-carboxylate derivatives (7 and 8), but rather to be 2-diazo-2,4-azulenequinone derivatives (5 and 6). The diazo carbons of both 5 and 6 show C-13 NMR signals at delta 66.3, and their carbonyl carbons resonate at 6 181.1, in good agreement with a 2-diazo-2,4-azulenequinone structure. The structures of diazo compounds 24 and 25, which we have previously reported,(1) are reexamined on the basis of the analysis of their C-13 NMR spectra. The contribution of a quinoid structure and a diazoazulenolate structure to 5, 6, 24, and 25 is discussed by comparison of their C-13 NMR spectral data with those of 4-diazo-2,5-cyclohexadien-1-one derivatives. It is concluded that the contribution of the quinoid structure is larger than that of the diazoazulenolate structure.DOI:10.1021/jo00123a031
-
作为产物:描述:2-氯-2,4,6-环庚三烯-1-酮 在 potassium tert-butylate 作用下, 以 四氢呋喃 、 xylene 为溶剂, 反应 33.5h, 生成 ethyl 2-oxo-2H-cyclohepta[b]furan-3-carboxylate参考文献:名称:(三苯基亚砷烷基)甲基环庚-2,4,6-三烯酮衍生物的合成,结构和反应性:杂多烯与活化乙炔的反应摘要:稳定的叶立德衍生物7a,b轴承环庚-2,4,6-三烯基和吸电子基团CO 2 Et和CN分别是通过与2-氯环庚-2,4,6-三烯酮在卜的存在相应的鉮甲基氧衍生物吨确定。化合物7a,b被分离为稳定的结晶化合物,不会经历水解即使在酸性条件下。X射线晶体分析表明,它们的As–O键距(7a为2.31Å,7b为2.39Å)低于范德华半径的总和(3.37Å),因此,砷和氧元素。为了构建一系列环庚环杂环并为了更好地理解一系列砷化钠,允许7a,b与二甲基碳杂杂环烯反应,异硫氰酸苯酯,二苯基碳二亚胺基和 异氰酸苯酯,在Wittig型反应中,随后进行电环化或正式的[8 + 2]型环加成消除 三苯ar硫醚 或者 氧化物 给 2 H-环庚[ b ]呋喃-2-硫酮, 它的 亚胺, 2-苯基亚氨基-2 H-环庚[ b ]吡咯, 和 2 H-环庚[ b ]呋喃-2-酮。另一方面,7a,b与乙炔二羧酸二甲酯 (DMAD)给DOI:10.1039/b205612g
文献信息
-
[8+2] Cycloaddition Reactions of 2H-Cyclohepta[b]furan-2-one with Acyclic 1,3-Dienes: A Facile Route to Novel Bicyclo [5.3.0] ring Systems作者:Vijay Nair、G Anilkumar、M.V Nandakumar、Bini Mathew、Nigam P RathDOI:10.1016/s0040-4039(97)01472-x日期:1997.9Novel [8+2] cycloaddition reactions of 3-ethoxy carbonyl 2H-cyclohepta[b]furan-2-one with acyclic 1,3-dienes are described. The potential application of this process in the synthesis of modified colchicinoids incorporating bicyclo [5.3.0] ring systems has also been studied. © 1997 Elsevier Science Ltd.
-
Synthesis and Analysis of Positive Inotropic Effects of 3-Substituted-2H-cyclohepta(b)furan-2-one Derivatives.作者:Masayuki YOKOTA、Takashi YANAGISAWA、Kazuhiro KOSAKAI、Shuichi WAKABAYASHI、Tsuyoshi TOMIYAMA、Masafumi YASUNAMIDOI:10.1248/cpb.42.865日期:——Several 3-substituted-2H-cyclohepta[b]furan-2-one derivatives were prepared and tested in vitro for positive inotropic character. Introduction of an isopropyl group at the 5-position of compound 8a caused an increase of PIC50 (negative logarithm of the dosage which increases the contractile force by 50%) from 4.48 to 5.10. Among the 5-isopropyl-8-alkoxy compounds, the isopropoxy compound 12f had the most potent activity with a PIC50 value of 5.99. Conversion of the ester group at the 3-position to a methylene group and of the alkoxy group at the 8-position to a substituted amino group caused a decrease in activity. The most active compound, 12f, was also found to have a weaker heart rate (HR)-increasing effect compared to milrinone and amrinone.
-
Cycloaddition reactions of 2-oxo-2H-cyclohepta[b]furan derivatives with dienes and dienophiles作者:Vijay Nair、M.V. Nandakumar、G. Anilkumar、Guenter K. EigendorfDOI:10.1016/s0040-4020(99)00100-3日期:1999.3Cycloaddition reactions of 2-oxo-2H-cyclohepta[b]furan derivatives participating as 8π and 4π components respectively towards different dienes and dienophiles are described. The observed reactivity and periselectivity have been rationalized by AM1 calculations.
-
The formation of azulene derivatives from 2H-cyclohepta[b]furan-2-one derivatives作者:T. Nozoe、K. Takase、T. Nakazawa、S. FukudaDOI:10.1016/s0040-4020(01)97748-8日期:——ethoxide or t-butyl-amine, giving the corresponding 1,2,3-trisubstituted azulene derivatives, respectively. The structures of these azulene derivatives are determined on the basis of chemical evidence, and spectral data. Considering the structural correlation between the starting 2H-cyclohepta[b]furan-2-ones and the azulene derivatives obtained, a reasonable reaction course, involving the heptafulvene-type
-
Cycloaddition reactions of 2-oxo-2H-cyclohepta[b]furan derivatives with arylacetylenes and the di-π-methane rearrangement of homobarrelene derivatives作者:Vijay Nair、Mecheril Valsan Nandakumar、Gopinathan Nair Anilkumar、Davis Maliakal、Mariappanadar Vairamani、Sripadi Prabhakar、Nigam P. RathDOI:10.1039/b005377p日期:——2-Oxo-2H-cyclohepta[b]furan derivatives participate in [4Â +Â 2]cycloaddition reactions with aryl acetylenes leading to homobarrelene derivatives and the latter undergo di-Ï-methane rearrangement resulting in complex carbocycles.
表征谱图
-
氢谱1HNMR
-
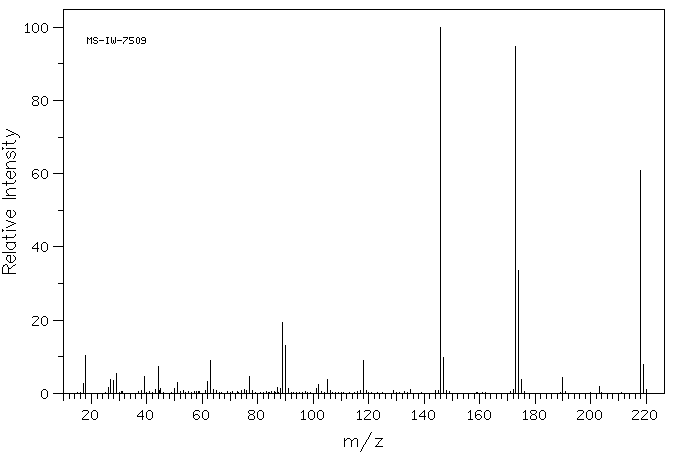
质谱MS
-
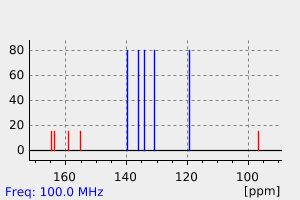
碳谱13CNMR
-
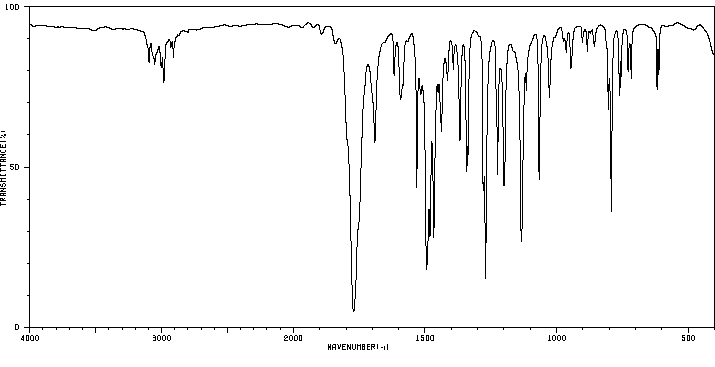
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
[2-二(2,4-二叔-丁基苯氧基)磷烷氧基-3,5-二叔-丁基-苯基]-氯-钯
N-(2-氰基乙基)-N-(2-吗啉-4-基乙基)-4-羰基-9,10-二氢-4H-苯并[4,5]环庚三烯并[1,2-b]呋喃-3-甲酰胺盐酸
N-(2-(二乙胺)乙基)-4-((2-(二乙胺)乙基)氨基)-9,10-二氢-4-羟基-4H-苯并(4,5)环庚三烯并[1,2-b]呋喃-3-甲酰胺
N,N-二乙基-4-羰基-9,10-二氢-4H-苯并[4,5]环庚三烯并[1,2-b]呋喃-3-甲酰胺
6H-2-氧杂薁-6-酮
5-甲基-2,3-二氢-7H-呋喃并[3,2-g]色烯-7-酮
5-异丙基-3-(甲氧羰基)-2H-环庚烷[b]呋喃-2-酮
3-乙酰基-2H-环庚并[b]呋喃-2-酮
3-(甲氧羰基)-2H-环庚[b]呋喃-2-酮
3,5,8-三甲基薁并[6,5-b]呋喃
2H-环戊并(b)呋喃-2-酮
2,7,8,9-四氢-6-甲基-9-亚甲基-2-氧代薁并[4,5-b]呋喃-3-甲醛
(8R)-1,5,8-三甲基-7,8-二氢-6H-薁并[7,6-D]呋喃-2-酮
(5aR,6S)-rel-(-)-5,5a,6,10-四氢-5a,6-二甲基-4H-苯并(5,6)环庚并(1,2-b)呋喃
(3R,5Z,7E)-3,25-二羟基-9,10-裂胆甾-5,7,10-三烯-23-酮
(2Z)-3-甲基-N-苯基-2H-环庚并[b]呋喃-2-亚胺
3-Methyl-8-hydroxy-2H-cycloheptafuran-2-on
5-bromo-1,3-di-tert-butyl-4,4,6-trimethyl-4H-cyclopenta[c]furan
2-Methyl-7-propylcycloheptafuran-8-on
4-methoxy-2,3,6-trimethyl-8,9-dihydro-furo[2,3-f]isoquinoline
3-(3,4-dihydro-2H-naphthalen-1-ylidenemethyl)furan
(4α,4aβ,5α,7α,7aα,8α,9β)-(+/-)-9-<<(1,1-dimethylethyl)dimethylsilyl>oxy>-4,4a,5,6,7,7a,8,9-octahydro-4a,8-dimethyl-4-(1-ethoxyethoxy)-5-<(2-methoxyethoxy)methoxy>-7-methoxyazuleno<6,5-b>furan
3-bromo-2-(4-fluorophenyl)-5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-b]furan
1-benzyl-3-ethyl-4,5,6,7-tetrahydroisobenzofuran
(E)-4,4,-dimethyl-3-(3,4-pentano-2-furyl)-2-pentenenitrile
(Z)-4,4-dimethyl-3-(3,4-pentano-2-furyl)-2-pentenenitrile
3,4,3',4'-tetrachloro-6,6'-o-phenylene-bis-pyran-2-one
4-(Furan-2-yl)-3,15-dioxatetracyclo[6.6.1.02,6.09,14]pentadeca-2(6),4,9,11,13-pentaene
4-Methoxy-2-methyl-6,7,8,9-tetrahydro-3-oxa-cyclohepta[e]inden-10-one
2-cyano-3-(5-isopropyl-2-oxo-2H-cyclohepta[b]furan-3-yl)-but-2-enedinitrile
3-cyano-2H-cycloheptafuran-2-one
7,7-Dimethyl-4,5,6,7,8,9-hexahydro-naphtho[2,3-c]furan-5-ol
5-Isopropyl-2-oxo-2H-cyclohepta[b]furan-3-carbonitrile
diethyl 1-phenyl-5-(5,6,7,8-tetrahydro-4H-cyclohepta[c]furan-3-yl)bicyclo[3.1.0]hexane-3,3-dicarboxylate
(4aS,7aS)-6,6-Dimethyl-8-methylene-4,4a,5,6,7,7a,8,9-octahydro-2-oxa-cyclopenta[f]azulene
4,5,6,7-tetrahydro-8H-cyclohepta[b]furan-8-one
ethyl 8-hydroxy-2-oxo-2H-cycloheptafuran-3-carboxylate
2,3-Pentamethylen-5-methoxy-benzofuran
8-Ethoxy-6-(4-methoxy-phenyl)-1,3-dimethyl-cyclohepta[c]furan-4-one
2-((3-((1-(furan-2-ylmethylene)-1H-inden-3-yl)methylene)-2,3-dihydro-1H-inden-1-ylidene)methyl)furan
isognididione
3β,9α,10β-Trihydroxyfuranoeremophilan
3-acetyl-7-isopropyl-cyclohepta[b]furan-2-one
2-(1-ethoxymethyleneamino)-4-(4-methoxyphenyl)-8,10-dimethyl-7-oxo-4H,7H-furo[3',4':6,7]cyclohepta[1,2-b]pyran-3-carbonitrile
furanoeremophilane-3β,9β,10β-triol