1-萘酚磷酸酯 | 4004-51-7
中文名称
1-萘酚磷酸酯
中文别名
Α-萘基磷酸酯;三(α-萘酚)磷酸酯;磷酸三-1-萘酚酯;-萘基磷酸酯
英文名称
tri-1-naphthyl phosphate
英文别名
naphthol phosphate;phosphoric acid tri-[1]naphthyl ester;Phosphorsaeure-tri-[1]naphthylester;Tri-(naphthyl-(1))-phosphat;Phosphorsaeure-tri-α-naphthylester;1-naphthalenol, phosphate (3:1);Tri-α-naphthyl-phosphat;trinaphthyl phosphate;1-naphthyl phosphate;1-Naphthol, phosphate (3:1);trinaphthalen-1-yl phosphate
CAS
4004-51-7
化学式
C30H21O4P
mdl
MFCD02110469
分子量
476.468
InChiKey
DBPYGJOORSXBMI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):8.7
-
重原子数:35
-
可旋转键数:6
-
环数:6.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2919900090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Fosse, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1903, vol. 136, p. 1007摘要:DOI:
-
作为产物:参考文献:名称:聚合物负载试剂:一种高效且简单的磷酸三芳基酯合成方法摘要:摘要 磷酰氯与不溶性聚合物负载的酚盐离子试剂在室温下在苯中反应,以优异的产率生成磷酸三芳基酯。通过简单的过滤和蒸发来分离纯产品是该方法的一个重要特点。DOI:10.1080/00397919408010211
文献信息
-
Palladium-Catalyzed C(sp<sup>2</sup>)–N Bond Cross-Coupling with Triaryl Phosphates作者:Zicong Chen、Xiangmeng Chen、Chau Ming SoDOI:10.1021/acs.joc.9b00703日期:2019.5.17The first general palladium-catalyzed amination of aryl phosphates is described. The combination of MorDalPhos with [Pd(π-cinnamyl)Cl]2 enables the amination of electron-rich, electron-neutral, and electron-poor aryl phosphates with a board range of aromatic, aliphatic, and heterocyclic amines. Common functional groups such as ether, keto, ester, and nitrile show an excellent compatibility in this
-
Nickel-Catalyzed Amination of Aryl Phosphates through Cleaving Aryl C–O Bonds作者:Jin-Hua Huang、Lian-Ming YangDOI:10.1021/ol201437g日期:2011.7.15amination of triaryl phosphates was achieved using a Ni(II)–(σ-Aryl) complex/NHC catalyst system in dioxane at 110 °C in the presence of NaH as base. Electron-neutral, -rich, and -deficient triaryl phosphates were coupled with a wider range of amine partners including cyclic and acyclic secondary amines, aliphatic primary amines, and anilines in good to excellent yields.
-
MICROWAVE-ASSISTED SYNTHESIS OF TRIARYL PHOSPHATES作者:A. D. Sagar、N. A. Shinde、B. P. BandgarDOI:10.1080/00304940009355923日期:2000.6Patented procedures are also available in the literaturei ‘’ for the synthesis of triaryl phosphates. Some of the reported methods have limitations such as (i) drastic reaction conditions,? (ii) liberation of hydrogen chloride which may cause corrosion problems in industrial reactors, (iii) tedious work-up, and (iv) long reaction times. In certain cases, pyridine or dialkylanilines have been used to neutralize磷酸三芳基酯由于可用作增塑剂、阻燃剂、润滑油添加剂和液压油而备受关注。它们是在路易斯酸催化剂的存在下,通过磷酰氯与酚类在高温(160-250”)下反应合成的。在室温下使用相转移催化剂'或通过使用聚合物负载的酚盐'。在文献中也可得到用于合成磷酸三芳基酯的专利方法。一些报道的方法有局限性,例如(i)剧烈的反应条件,?(ii) 氯化氢的释放可能导致工业反应器中的腐蚀问题,(iii) 繁琐的后处理,以及 (iv) 反应时间长。在某些情况下,吡啶或二烷基苯胺已被用于中和释放的氯化氢。高温通常会产生许多副产品。增塑剂级磷酸三芳基酯必须具有非常高的纯度,并且获得的产品必须在低压下蒸馏以进行纯化。工业规模的低压蒸馏会产生严重的问题。
-
BENZOXAZOLE AND BENZOTHIAZOLE COMPOUNDS AND METHODS THEREFOR申请人:Chauhan Yogendrasinh Bharatsinh公开号:US20080081913A1公开(公告)日:2008-04-03A compound of Formula (I): wherein R 1 is selected from the group consisting of an aliphatic functionality having 2 to 12 carbons, an aromatic functionality having 3 to 20 carbons, and a cycloaliphatic functionality having 3 to 20 carbons; with the proviso that R 1 is not —C 6 H 5 or —NH—C 10 H 7 ; R 2 and R 3 are independently selected from the group consisting of a hydroxyl group, a halogen atom, an aliphatic functionality having 2 to 12 carbons, an aromatic functionality having 3 to 20 carbons, and a cycloaliphatic functionality having 3 to 20 carbons; X is either an oxygen atom or a sulfur atom; “n” has a value of 0 to 4; and “m” has a value of 0 to 3.
-
Photolyses of Derivatives of Naphthyl and Anthryl Phosphates and Methylphosphonates作者:Mitsunobu Nakamura、Koichi Sawasaki、Yoshiki Okamoto、Setsuo TakamukuDOI:10.1246/bcsj.68.3189日期:1995.11methylphosphonate underwent intramolecular (4+4) photocycloaddition between two anthryl groups. The fluorescence spectra of the naphthyl derivatives had two emission bands ascribed to an intramolecular excimer and a monomer, but the fluorescence spectra of the anthryl derivatives had only a monomer emission band. These photoluminescence behaviors are closely related to the reactivities of the compounds.在乙腈中紫外线照射下,磷酸三-1-萘酯和甲基膦酸二-1-萘酯发生分子内重排和ipso偶联,分别得到1,2'-联萘-1'-醇和1,1'-联萘。在三(4-甲氧基-1-萘基)磷酸酯和双(4-甲氧基-1-萘基)甲基膦酸酯在甲醇中的光解中,4,4'-二甲氧基-1,1'-联萘,1',4,4生成'-三甲氧基-1,2'-联萘和2,4,4'-三甲氧基-1,1'-联萘。Tri-9-anthryl phosphate 和 di-9-anthrylmethylphosphonate 经历了两个蒽基之间的分子内 (4+4) 光环加成。萘基衍生物的荧光光谱有两个来自分子内准分子和单体的发射带,而蒽基衍生物的荧光光谱只有一个单体发射带。
表征谱图
-
氢谱1HNMR
-
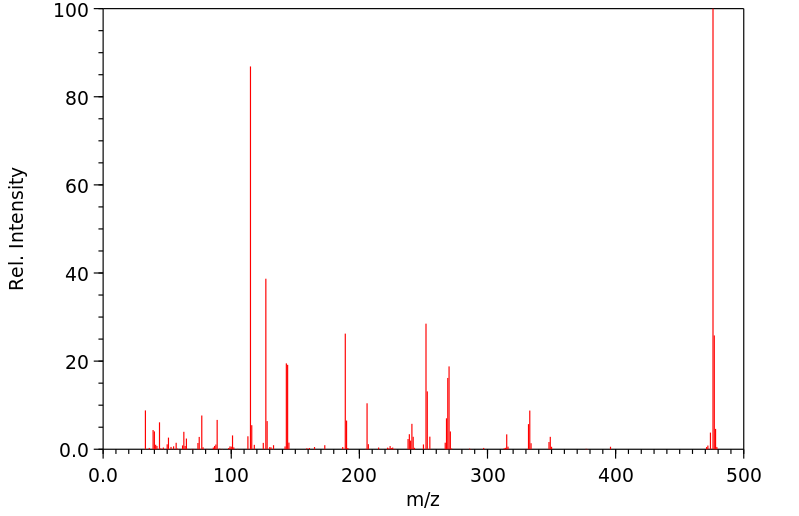
质谱MS
-
碳谱13CNMR
-
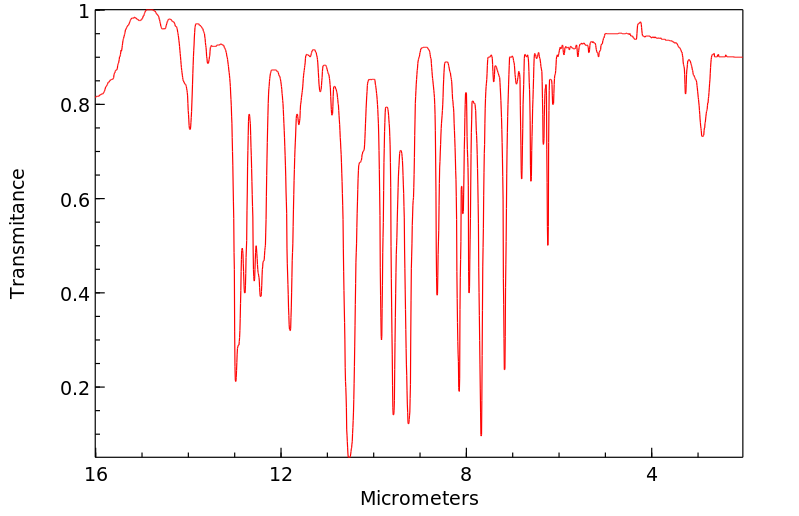
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11bR,11''bR)-2,2''-[氧双(亚甲基)]双[4-羟基-4,4''-二氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11aR)-10,11,12,13-四氢-5-羟基-3,7-二-1-萘-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂磷杂八环
鲸蜡基磷酸-鲸蜡基磷酸二乙醇胺
高氯酸N,N,N',N',N'',N'',N''',N'''-八甲基二磷四酰胺(1:1:2)锂
非对称二乙基二(二甲基胺基)焦磷酸酯
非4-烯-5-基二苯基磷酸酯
雷公藤甲素O-甲基磷酸酯二苄酯
阿扎替派
间苯二酚双[二(2,6-二甲基苯基)磷酸酯]
锌四戊基二(磷酸酯)
银(1+)二苄基磷酸酯
铵4-(2-甲基-2-丁炔基)苯基4-(2-甲基-2-丙基)苯基磷酸酯
铵2-乙基己基磷酸氢酯
铵2,3-二溴丙基磷酸酯
钾二己基磷酸酯
钾二十烷基磷酸酯
钾二乙基磷酸酯
钾二(8-甲基壬基)磷酸酯
钾[5,7,7-三甲基-2-(1,3,3-三甲基丁基)辛基]磷酸酯
钾2-己基癸基磷酸酯
钴(2+)十三烷基磷酸酯
钡4,4-二乙氧基-2,3-二羟基丁基磷酸酯
钡1,3-二羟基-2-丙基磷酸酯
钠辛基氢磷酸酯
钠癸基氢磷酸酯
钠异丁基氢磷酸酯
钠二苄基磷酸酯
钠二戊基磷酸酯
钠二(十八烷基)磷酸酯
钠二(2-丁氧乙基)磷酸酯
钠O,O-二乙基磷酰蔷薇l烯酸酯
钠4-氨基苯基氢磷酸酯水合物(1:1:1)
钠3,6,9,12,15-五氧杂二十八碳-1-基氢磷酸酯
钠2-乙氧基乙基磷酸酯
钠2,3-二溴丙基磷酸酯
钛酸酯偶联剂NDZ-201
钙敌畏
钙二钠氟-二氧代-氧代膦烷碳酸盐
钙3,9-二氧代-2,4,8,10-四氧杂-3lambda5,9lambda5-二磷杂螺[5.5]十一烷3,9-二氧化物
野尻霉素6-磷酸酯
酸式磷酸戊酯
酚酞单磷酸酯
酚酞单磷酸环己胺盐
酚酞二磷酸四钠盐
酚酞二磷酸四钠
辛基磷酸酯
辛基二氯膦酸酯
辛基二氯丙基磷酸酯
辛基二丙基磷酸酯
赤藓糖醇4-磷酸酯