硫酸锆四水 | 7446-31-3
分子结构分类
中文名称
硫酸锆四水
中文别名
硫酸锆四水合物
英文名称
Zirconium sulfate tetrahydrate
英文别名
zirconium(4+);disulfate;tetrahydrate
CAS
7446-31-3
化学式
H8O12S2Zr
mdl
——
分子量
355.4
InChiKey
NLOQZPHAWQDLQW-UHFFFAOYSA-J
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:410 °C
-
密度:3.22
-
溶解度:极易溶于H2O
-
暴露限值:ACGIH: TWA 5 mg/m3; STEL 10 mg/m3NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-5.98
-
重原子数:15
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:181
-
氢给体数:4
-
氢受体数:12
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:28332980
-
危险品运输编号:UN 3260 8/PG 3
-
WGK Germany:3
-
RTECS号:LV1950000
-
危险类别:6.1
SDS
Section 1: Product Identification
Chemical Name: Zirconium (IV) sulfate tetrahydrate (99.99+%-Zr) PURATREM
CAS Registry Number: 7446-31-3
Formula: Zr(SO4)2.4H2O
EINECS Number: 238-694-4
Chemical Family: metal sulfate
Synonym: Sulfuric acid, zirconium(4+) salt (2:1), Zirconium sulfate
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 7446-31-3 100% 5mg/m3 (as Zr) 5mg/m3 (as Zr)
Section 3: Hazards Identification
Irritating to skin, eyes and respiratory tract. This material partially hydrolyzes in water to form dilute sulfuric
Emergency Overview:
acid, a strong irritant.
Primary Routes of Exposure: Ingestion, inhalation, skin, eyes
Eye Contact: Dust is a moderate to severe irritant to the eyes.
Skin Contact: Causes irritation to skin. Symptoms include redness, itching, and pain.
Inhalation: Inhalation of dust may irritate the respiratory tract. Symptoms may include coughing, shortness of breath.
Ingestion: Ingestion may lead to nausea, vomiting and diarrhea.
Acute Health Affects: Acidic behavior causes irritation of skin, eyes and respiratory and gastrointestinal tracts.
Chronic Health Affects: No information available on long-term chronic effects.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: not applicable
Autoignition Temperature: none
Explosion Limits: none
Extinguishing Medium: none required
If involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure self-contained
Special Fire Fighting Procedures:
breathing apparatus and full protective clothing.
Hazardous Combustion and If involved in a fire this material may emit toxic fumes of sulfur oxides.
Decomposion Products:
Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards.
SECTION 6: Accidental Release Measures
Spill and Leak Procedures: Small spills can be mixed with powdered sodium carbonate or ground limestone and swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store material in a tightly sealed bottle away from moisture.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear appropriate chemical resistant gloves and protective clothing.
Ventilation: If possible, handle the material in an efficient fume hood.
In the absence of adequate ventilation a respirator should be worn. The use of a respiratory requires a
Respirator:
Respirator Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: If possible, handle the material in an efficient fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: white xtl.
Molecular Weight: 283.33 (355.39)
Melting Point: 100°C (-3H2O)
Boiling Point: no data
Vapor Pressure: not applicable
Specific Gravity: no data
Odor: none
Solubility in Water: soluble
SECTION 10: Stability and Reactivity
Stability: air and moisture stable
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: none
Incompatibility: Strong bases
Decomposition Products: In water, dilute sulfuric acid
SECTION 11: Toxicological Information
Oral (rat); LD50: 3500 mg/kg. Intraperitoneal (rat); LD50: 175 mg/kg. Subcutaneous (rat); LDLo: 500 mg/kg.
RTECS Data:
Intratesticular (rat); TDLo: 22667 ug/kg. Mouse Lymphocyte; Unscheduled DNA synthesis: 20 umol/L.
Carcinogenic Effects: no data
Mutagenic Effects: no data
Tetratogenic Effects: no data
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to local, state and federal regulations.
SECTION 14: Transportation
Shipping Name (CFR): Non-hazardous
Hazard Class (CFR): NA
Additional Hazard Class (CFR): NA
Packaging Group (CFR): NA
UN ID Number (CFR): NA
Shipping Name (IATA): Non-hazardous
Hazard Class (IATA): NA
Additional Hazard Class (IATA): NA
Packaging Group (IATA): NA
UN ID Number (IATA): NA
SECTION 15: Regulatory Information
TSCA: Listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:参考文献:名称:Process for the purification of zirconium compounds摘要:本发明涉及制备锆化合物的方法,经焙烧后形成氧化锆。该锆化合物的制备方法是将氨源添加到水合硫酸锆溶液中,使溶液pH在0.1至2.5范围内,优选在1.0至2.0范围内。从溶液中沉淀出的锆化合物呈结晶状,易于通过过滤收集,并且具有低水平的金属杂质。因此,本发明的方法可用于纯化锆化合物。本发明还包括锆化合物和纯化锆化合物(包括氧化锆)的方法。公开号:US04822575A1
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
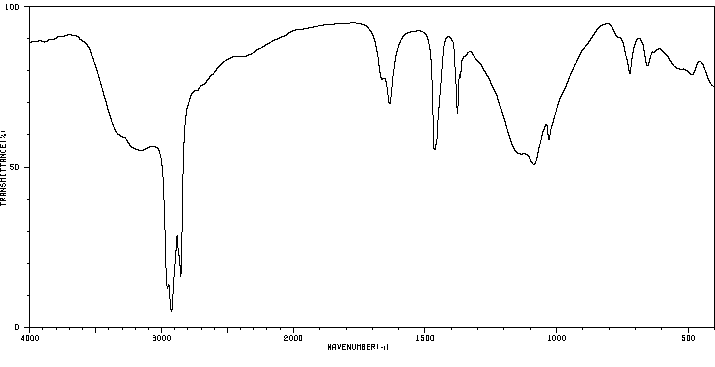
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氯酸镉
高氯酸锰六水合物
高氯酸锰六水合物
高氯酸锌,六水合物
高氯酸银水合物
高氯酸银
高氯酸铬盐(3:1)
高氯酸铬六水合物
高氯酸铜
高氯酸钴盐(2:1)
高氯酸汞(II)六水合物
高氯酸汞
高氯酸亚铁
高氯酸亚汞
颜料黄36
颜料绿26
顺式-二氨基二硝酸基铂
镍钼氧化物
镍(II)高溴酸盐
镍(2+)亚硒酸盐
镉二亚硝酸盐
锰碲三氧化物
锰亚硒酸盐
锰-硝酸(1:1)
锌铬黄
锌氮烷硫酸盐
锌亚硝酸盐
锌二氢二磷酸酯
锆(2+)氯化物氢氧化物(1:1:1)
铵银硫酸盐
铵铜砷酸盐
铵金(1+)亚硫酸酯(1:1:1)
铱(3+)硫酸盐(2:3)
铬铁矿,亚铬酸盐
铬酸锰
铬酸锌氧化物单水合物
铬酸钴
铬酸洗液
铬酸三铜四氢氧化物
铬酸三铜二氧化物
铬硫酸盐六水合物
铪四水合物
铜铁锰钾锌五氯化物碘化物二硫酸盐
铜氧代氧基磺酰基硫酸盐
铜-锌硫酸盐络合物
铜(1+)高氯酸酯
铁钠二硫酸盐
铁(3+)三[羟基(氧代)二氧化铬]
铁(2+)硫酸酯
铁(2+)氧代硅烷二醇酸酯