2-(furan-2-yl)-4,6-di-phenyl-1,3,5-triazine | 7753-01-7
中文名称
——
中文别名
——
英文名称
2-(furan-2-yl)-4,6-di-phenyl-1,3,5-triazine
英文别名
2-(furan-2-yl)-4,6-diphenyl-1,3,5-triazine;Furyl-diphenyl-s-triazin;2-(2-Furyl)-4,6-diphenyl-1,3,5-triazine
CAS
7753-01-7
化学式
C19H13N3O
mdl
——
分子量
299.332
InChiKey
MAVXDEAJWADQDI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:194-195 °C
-
沸点:528.4±42.0 °C(Predicted)
-
密度:1.208±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:23
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:4
SDS
反应信息
-
作为产物:参考文献:名称:在可循环利用的OMS-2催化剂上通过酒精和苄am的需氧氧化无碱合成1,3,5-三嗪摘要:以4Na 2 SO 4 ·2H 2 O 2 ·NaCl为还原剂,成功制备了比表面积大,混合价高的氧化锰八面体分子筛(OMS-2)。OMS-2对由苄醇和苯甲idine有氧氧化合成1,3,5-三嗪具有极好的催化能力。甲基苯,DMF和DMSO也可以用作底物,与苯甲idine在异质条件下反应生成三嗪。该催化系统具有无碱条件,广泛的底物范围,高化学选择性,操作简便,催化剂可回收性以及O 2作为绿色氧化剂的利用。DOI:10.1016/j.catcom.2019.05.005
文献信息
-
iEDDA Reaction of the Molecular Iodine-Catalyzed Synthesis of 1,3,5-Triazines via Functionalization of the sp<sup>3</sup> C–H Bond of Acetophenones with Amidines: An Experimental Investigation and DFT Study作者:Abhishek R. Tiwari、Shilpa R. Nath、Kaustubh A. Joshi、Bhalchandra M. BhanageDOI:10.1021/acs.joc.7b02313日期:2017.12.15Diels–Alder (iEDDA)-type reaction to synthesize 1,3,5-trizines from acetophenones and amidines. The use of molecular iodine in a catalytic amount facilitates the functionalization of the sp3 C–H bond of acetophenones. This is a simple and efficient methodology for the synthesis of 1,3,5-triazines in good to excellent yields under transition-metal-free and peroxide-free conditions. The reaction is believed to
-
Iridium-catalyzed cascade dehydrogenation, ring-closure reaction leading to 2,4,6-triaryl-1,3,5-triazines作者:Gang Shi、Fei He、Youxin Che、Caihua Ni、Ying LiDOI:10.1134/s1070363216020304日期:2016.2An efficient iridium-catalyzed dehydrogenation, ring-closure reaction, has been developed via a cascade sequence, in which [Cp*IrI2]2/Xantphos proved to be the most efficient catalyst for the synthesis of 2,4,6-triaryl-1,3,5-triazines from stable aryl-substituted alcohols and amidines. It was the first case of iridium catalyst successful application in such transformation.
-
NIS-catalyzed oxidative cyclization of alcohols with amidines: a simple and efficient transition-metal free method for the synthesis of 1,3,5-triazines作者:Abhishek R. Tiwari、Akash T.、Bhalchandra M. BhanageDOI:10.1039/c5ob01835h日期:——An efficient method for the synthesis of 1,3,5-triazines by NIS-catalyzed oxidative cyclization of alcohols with amidines has been developed. The reaction works smoothly under transition-metal free and phosphine-free conditions to afford a wide range of 1,3,5-triazine derivatives in moderate to good yields. The synthetic methodology was achieved via in situ oxidation of alcohols to aldehydes.
-
An efficient ruthenium-catalyzed dehydrogenative synthesis of 2,4,6-triaryl-1,3,5-triazines from aryl methanols and amidines作者:Feng Xie、Mengmeng Chen、Xiaoting Wang、Huanfeng Jiang、Min ZhangDOI:10.1039/c3ob42589d日期:——By using [RuCl2(p-Cymene)]2/Cs2CO3 as an efficient catalyst system, the readily available, inexpensive aryl methanols were firstly employed for dehydrogenative synthesis of aryl substituted 1,3,5-triazine derivatives. Due to the inherent stability of alcohols in contrast with aldehydes, our synthetic protocol is adaptable to a broad substrate scope, there is no need for stringent protection during
-
Polythene glycol (PEG) as a reusable solvent system for the synthesis of 1,3,5-triazines via aerobic oxidative tandem cyclization of benzylamines and N-substituted benzylamines with amidines under transition metal-free conditions作者:Abhishek R. Tiwari、Bhalchandra M. BhanageDOI:10.1039/c5gc01884f日期:——A green and highly efficient protocol for the syntheis of 1,3,5-triazines from benzylamines and N-substituted benzylamines amidines in PEG-600 has been developed firstly. This protocol is transition-metal free, phosphine ligand...
表征谱图
-
氢谱1HNMR
-
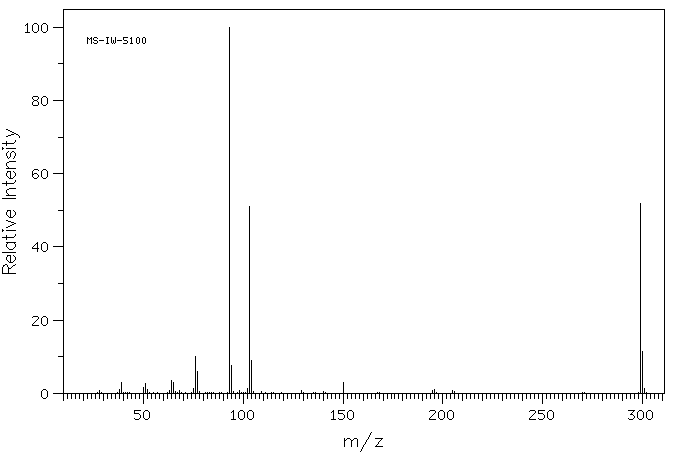
质谱MS
-
碳谱13CNMR
-
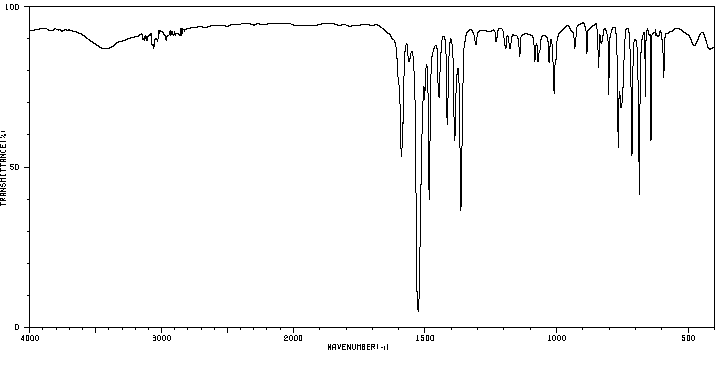
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4