4-Dimethylamino-azobenzol-4'-aldehyd | 39208-00-9
中文名称
——
中文别名
——
英文名称
4-Dimethylamino-azobenzol-4'-aldehyd
英文别名
Benzaldehyde, 4-((4-(dimethylamino)phenyl)azo)-;4-[[4-(dimethylamino)phenyl]diazenyl]benzaldehyde
CAS
39208-00-9
化学式
C15H15N3O
mdl
——
分子量
253.304
InChiKey
CVURSMYMOJMTTI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178-179 °C
-
沸点:434.3±30.0 °C(Predicted)
-
密度:1.08±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:45
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
反应信息
-
作为反应物:描述:4-Dimethylamino-azobenzol-4'-aldehyd 在 盐酸羟胺 、 sodium acetate 作用下, 以 甲醇 为溶剂, 反应 1.0h, 以83%的产率得到[4-(4-dimethylaminophenylazo)]benzaldehyde oxime参考文献:名称:Copper-Free “Click” Modification of DNA via Nitrile Oxide−Norbornene 1,3-Dipolar Cycloaddition摘要:Nitrile oxides react smoothly and rapidly with norbornene-modified DNA in a copper-free click reaction. The reaction allows high density functionalization of oligodeoxyribonucleotides (ODNs) with a large variety of molecules directly on solid supports and even in synthesizers without the need for an additional catalyst.DOI:10.1021/ol9005322
-
作为产物:参考文献:名称:由 3-Guaiazulenyl 基团和偶氮苯单元稳定的新型单碳鎓离子化合物的制备、结构和性质:三种离域 π 电子系统的比较研究摘要:在六氟磷酸的存在下,愈创甘油醚(=7-异丙基-1,4-二甲基甘菊烯)与 4'-羟基偶氮苯-4-甲醛在甲醇中在 25 °C 下反应 2 小时,产率高达 94%。DOI:10.1246/bcsj.82.879
文献信息
-
Effect of nitro substitution of azo-chalcone derivatives nano film on electrical memory properties作者:Quan Liu、Jiu-Fu Lu、Ling-Xia JinDOI:10.1080/24701556.2020.1844236日期:——photoelectric properties. The active layer was further prepared by solution spin coating, and the morphology of the film was characterized by AFM. The electrical memory performance of the device is studied. It is found that the device has the nonvolatile binary write once read many (WORM) memory performances, and the threshold voltage of the device prepared by introducing nitro group into the molecule is摘要 硝基取代的偶氮查尔酮衍生物3-(4-(二甲氨基)苯基)二氮烯基)苯基)-1-(4-硝基苯基)prop-2-en-1-one( AZOC-4N )和参考化合物3-( 4-(二甲氨基)苯基)二氮烯基)苯基)-1-苯基丙-2-烯-1-酮( AZOC-H )通过克莱森施密特反应合成。采用热重法、紫外-可见光法和循环伏安法研究在共轭分子AZOC-4N中引入硝基的影响关于光电特性。通过溶液旋涂进一步制备活性层,并通过AFM表征薄膜的形貌。研究了器件的电记忆性能。发现该器件具有非易失性二进制写入一次读取多次(WORM)存储性能,分子中引入硝基制备的器件阈值电压较低—1.4 V。 最后,器件的电存储性能通过理论计算进行分析。
-
New D-π-A chromophores incorporating (5,5-dimethylcyclohex-2-en-1-ylidene)- or (6-methyl-4H-pyran-4-ylidene)-malononitrile moiety作者:D. G. Slobodinyuk、A. N. Vasyanin、I. V. Lunegov、E. V. Sklyaeva、G. G. AbashevDOI:10.1007/s11172-022-3417-2日期:2022.2New D-π-A-type chromophores that simultaneously incorporate N,N-dimethylaminophenyl moiety as the terminal electron-donating group and either (5,5-dimethylcyclohex-2-en-1-ylidene)malononitrile (DCM-1) or (6-methyl-4H-pyran-4-ylidene)malononitrile (DCM-2) unit as the terminal electron-withdrawing group were synthesized. Vinylene moiety and azo group served as the π-spacer. A comparative analysis of optical and electrochemical properties of the synthesized chromophores revealed that the replacement of the DCM-1 unit with the DCM-2 moiety led to chromophore band gap broadening and a decrease in the molar extinction coefficient value; while the replacement of the DCM-1 unit with the DCM-2 one in the chromophores bearing the vinylene π-spacers led to a sharp increase in the fluorescence quantum yield.
-
Azobenzene‐Based Colorimetric Chemosensors for Rapid Naked‐Eye Detection of Mercury(II)作者:Xiaohong Cheng、Qianqian Li、Conggang Li、Jingui Qin、Zhen LiDOI:10.1002/chem.201003275日期:2011.6.20Two new highly selective colorimetric chemosensors for Hg2+, based on azobenzene and highly selective Hg2+‐promoted deprotection of a dithioacetal have been designed and synthesized. In the presence of as little as 20 μM Hg2+, the sensors change their color from light yellow to deep red, which can easily be observed by the naked eye. The underlying signaling mechanism is intramolecular charge transfer
-
Perez-Moreno, Javier; Zhao, Yuxia; Clays, Koen, Journal of the American Chemical Society, 2009, vol. 131, p. 5084 - 5093作者:Perez-Moreno, Javier、Zhao, Yuxia、Clays, Koen、Kuzyk, Mark G.、Shen, Yuquan、et al.DOI:——日期:——
-
Fluorescence Quenchers for Hydrazone and Oxime Orthogonal Bioconjugation作者:Pete Crisalli、Armando R. Hernández、Eric T. KoolDOI:10.1021/bc300344b日期:2012.9.19We describe the synthesis and properties of new fluorescence quenchers containing aldehyde, hydrazine, and aminooxy groups, allowing convenient bioconjugation as oximes or hydrazones. Conjugation to oligonucleotides proceeded in high yield with aniline as catalyst. Kinetics studies of conjugation show that, under optimal conditions, a hydrazine or aminooxy quencher can react with aldehyde-modified DNA to form a stable hydrazone or oxime adduct in as little as five minutes. The resulting quencher-containing DNAs were assessed for their ability to quench the emission of fluorescein in labeled complements and compared to the commercially available dabcyl and Black Hole Quencher 2 (BHQ2), which were conjugated as phosphoramidites. Results show that the new quenchers possess slightly different absorbance properties compared to dabcyl and are as efficient as the commercial quenchers in quenching fluorescein emission. Hydrazone-based quenchers were further successfully incorporated into molecular beacons and shown to give high signal to background ratios in single nucleotide polymorphism detection in vitro. Finally, aminooxy and hydrazine quenchers were applied to quenching of an aldehyde-containing fluorophore associated with living cells, demonstrating cellular quenching within one hour.
表征谱图
-
氢谱1HNMR
-
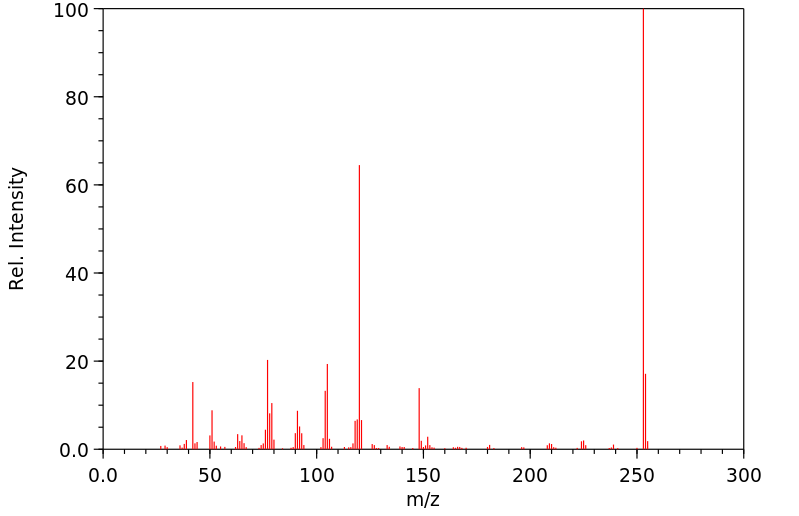
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黑洞猝灭剂-2,BHQ-2ACID
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S,24S)-
颜料橙61
阿利新黄GXS
阳离子红X-GTL
阳离子红5BL
阳离子橙RN
阳离子橙GLH
间甲基红
镨(3+)丙烯酰酸酯
镍酸酯(1-),[3-羟基-4-[(4-甲基-3-硫代苯基)偶氮]-2-萘羧酸根(3-)]-,氢
锂3-({4-[(4-羟基苯基)偶氮]-5-甲氧基-2-甲基苯基}偶氮)苯磺酸酯
钴,[二[m-[[1,2-二苯基-1,2-乙二酮1,2-二(肟酸根-kO)](2-)]]四氟二硼酸根(2-)-kN1,kN1',k2,kN2']-,(SP-4-1)-
钠5-氯-2-羟基-3-[(2-羟基-4-{[(4-甲基苯基)磺酰基]氧基}苯基)偶氮]苯磺酸酯
钠5-[[3-[[5-[[4-[[[4-[(4,5-二氢-3-甲基-5-氧代-1H-吡唑-4-基)偶氮]苯基]氨基]羰基]苯基]偶氮]-2,4-二羟基苯基]偶氮]-4-羟基苯基]偶氮]水杨酸盐
钠4-[(4-氨基苯基)偶氮]苯甲酸酯
钠4-[(4-{[4-(二乙基氨基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-[(4-{[2-羟基-5-(2-甲基-2-丙基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-({3-甲氧基-4-[(4-甲氧基苯基)偶氮]苯基}偶氮)苯磺酸酯
钠3-[4-(2-羟基-5-甲基-苯基)偶氮苯基]偶氮苯磺酸酯
钠3-({5-甲氧基-4-[(4-甲氧基苯基)偶氮]-2-甲基苯基}偶氮)苯磺酸酯
钠3-({4-[(4-羟基-2-甲基苯基)偶氮]-3-甲氧基苯基}偶氮)苯磺酸酯
金莲橙O
重氮基烯,苯基[4-(三氟甲基)苯基]-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(Z)-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(E)-
重氮基烯,[4-[(2-乙基己基)氧代]-2,5-二甲基苯基](4-硝基苯基)-
重氮基烯,1,2-二(4-丙氧基苯基)-,(1E)-
重氮基烯,(2-氯苯基)苯基-
酸性金黄G
酸性棕S-BL
酸性媒染棕
酸性媒介棕6
酸性媒介棕48
酸性媒介棕4
酸性媒介棕24
邻氨基偶氮甲苯
达布氨乙基甲硫基磺酸盐
赛甲氧星
茴香酸盐己基
茜素黄 R 钠盐
苯重氮化,2-甲氧基-5-甲基-4-[(4-甲基-2-硝基苯基)偶氮]-,氯化
苯酰胺,4-[4-(2,3-二氢-1,4-苯并二噁英-6-基)-5-(2-吡啶基)-1H-咪唑-2-基]-
苯酚,4-(1,1-二甲基乙基)-2-(苯偶氮基)-
苯酚,2-甲氧基-4-[(4-硝基苯基)偶氮]-
苯胺棕
苯胺,4-[(4-氯-2-硝基苯基)偶氮]-
苯磺酸,3,3-6-(4-吗啉基)-1,3,5-三嗪-2,4-二基二亚氨基2-(乙酰基氨基)-4,1-亚苯基偶氮二-,盐二钠
苯磺酸,2-[(4-氨基-2-羟基苯基)偶氮]-
苯甲酸,5-[[4-[(乙酰基氨基)磺酰]苯基]偶氮]-2-[[3-(三氟甲基)苯基]氨基]-(9CI)