5-phenyl-1,4-oxathian-2-one | 32863-49-3
中文名称
——
中文别名
——
英文名称
5-phenyl-1,4-oxathian-2-one
英文别名
1,4-Oxathian-2-one, 5-phenyl-
CAS
32863-49-3
化学式
C10H10O2S
mdl
——
分子量
194.254
InChiKey
WUHIDFYVWSXODK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:51.6
-
氢给体数:0
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (2-Hydroxy-1-phenyl-ethylsulfanyl)-acetic acid 87792-25-4 C10H12O3S 212.269
反应信息
-
作为产物:描述:2-溴-2-苯基乙醛二乙缩醛 在 对甲苯磺酸 盐酸 、 sodium hydroxide 、 sodium tetrahydroborate 作用下, 以 甲醇 、 水 、 苯 为溶剂, 反应 1.0h, 生成 5-phenyl-1,4-oxathian-2-one参考文献:名称:Koskimies, Jorma K., Journal of the Chemical Society. Perkin transactions II, 1985, p. 1449 - 1456摘要:DOI:
文献信息
-
LiBr catalyzed solvent-free ring expansion of epoxides to 1,4-oxathian-2-ones with α-mercaptocarboxylic acids作者:Atul K. Singh、Ankita Rai、Lal Dhar S. YadavDOI:10.1016/j.tetlet.2011.05.010日期:2011.7An efficient and rapid (10–20 min) one-pot synthesis of chemically and pharmaceutically interesting 1,4-oxathian-2-ones is reported. The protocol involves LiBr catalyzed regioselective ring-opening–ring-closing reaction cascade of terminal epoxides with α-mercaptocarboxylic acids at rt under solvent-free conditions. Recycling of the catalyst, atom economy, and formation of water as the only by-product
-
Synthesis of 1,4-oxathian-2-ones by triton B-catalyzed one-pot reaction of epoxides with ethyl mercaptoacetate作者:Lassaad Wechteti、Nejib Hussein Mekni、Moufida Romdhani-YounesDOI:10.1515/hc-2017-0273日期:2018.8.28Abstract A rapid one-pot reaction of epoxides with ethyl mercaptoacetate furnishing 1,4-oxathian-2-ones in the presence of a catalytic amount of eco-friendly triton B is reported. High regioselectivity is due to the nucleophilic attack on the less sterically hindered carbon atom of the aliphatic unsymmetrical epoxide.
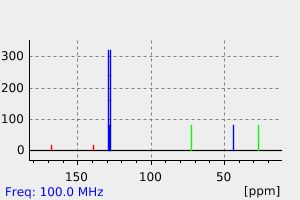
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
硫代羟基乙酸酐
二乙氧基-(1,4-恶噻烷-3-基硫基)-硫代膦烷
N,N-二甲基-1-十八烷基胺与1,2-氧杂硫羟基烷-2,2-二氧化物的反应产物
6-异丙基-1,4-恶噻烷-2-酮
5-甲基-3-苯基-2-氧杂-4-硫杂-二环[3.3.1]壬烷
5-异丙基-2-甲基-1,3-氧硫杂环已烷
4,4-二氢-4-亚氨基-1,4-氧硫杂环己烷 4-氧化物
3,4-环氧四氢噻吩-1,1-二氧化物
2-甲基-4-正丙基-1,3-氧硫杂环己烷
2-甲基-1,4-氧硫杂环已烷4,4-二氧化物
2-甲基-1,3-氧硫杂环已烷
2-异丙基-5-甲基-1,3-氧硫杂环已烷
2,6-二甲基-1,4-氧硫杂环己烷
2,6-二甲基-1,3-氧硫杂环已烷
1-甲基-6-氧杂-3-硫杂双环[3.1.0]己烷3,3-二氧化物
1-氧杂-4-硫杂螺[4.5]癸烷-2-甲醇氨基甲酸酯
1,4-氧硫杂环已烷4-氧化物
1,4-恶噻烷-2-酮
1,4-噻烷-1,1-二氧
1,4-噻烷
1,4-丁磺酸内酯
1,3-氧硫杂环已烷
1,2-氧杂硫烷,3,3,4,4,5,5,6,6-八氟-,2,2-二氧化
3-fluoro-1,4-butanesultone
methyl 2,6-anhydro-4-O-benzyl-3-O-methyl-2-thio-β-L-mannopyranoside
2,2,3,3-Tetrafluor-1,4-oxathian
(1R,4S,5R)-4-methyl-6,8-dioxa-3-thiabicyclo[3.2.1]octane
2,2-Dichloro-4-(3,3-dichloro-tetrahydro-furan-2-yloxy)-butyraldehyde
2-propoxymethyl-[1,4]oxathiane 4,4-dioxide
3,7-Dioxa-10-thia <3,3,3> propellan
3,5-Dimethyl-3,5-dichlormethyl-1,4-oxathian
cis-1,5-Dimethyl-3-oxa-7-thiabicyclo<3.3.0>octan
2-(2-Methyl-1-propenyl)-1,3-oxathiolane
Lithium(1+)6,6-dioxo-2-oxa-6lambda6-thiaspiro[3.4]octane-8-carboxylate
5-Methyl-6-methylene-[1,4]oxathian-2-one
(1'R)-2-<1',2'-Dimethyl-1'-hydroxypropyl>hexahydro-4,4,7-trimethyl-4H-1,3-benzoxathiin
2,5-Anhydro-3,4-dehydro-3,4-desoxy-1,6-thioanhydro-D-glucit
(10S,11S)-10,11-Dimethyl-1,9-dioxa-3,7-dithia-cycloundecane-2,8-dithione
(5S,6S)-6-methyl-5-propan-2-yl-1,3-oxathian-4-one
4-Methyl-1,2-oxathian-2-oxid
2-(methylthio)-1,3-oxathiane
2-ethyl-[1,4]oxathiane 4,4-dioxide
5-[(E)-5-iodopent-3-enyl]-1,4-oxathian-2-one
5-Methyl-1,2-oxathian-2-oxid
Xjzsoywhxcrhsd-aoooyvtpsa-
(1'S)-2-<1',2'-Dimethyl-1'-hydroxypropyl>hexahydro-4,4,7-trimethyl-4H-1,3-benzoxathiin
(S)-2-((2R,6S)-4,4-Dioxo-7a-phenylsulfanyl-6-propyl-hexahydro-1,5-dioxa-4λ6-thia-inden-2-yl)-propionic acid methyl ester
6-methyl-8-hydroxymethylimino-3,7-dioxa-9-thiabicyclo<4.3.0>nonane