(4aS)-trans-decahydroquinoline | 22160-37-8
中文名称
——
中文别名
——
英文名称
(4aS)-trans-decahydroquinoline
英文别名
(4aS)-trans-Decahydrochinolin;trans-(+)-decahydro-quinoline;trans-Decahydrochinolin;trans-Dekahydrochinolin;(4aR,8aS)-decahydroquinoline;(4aS,8aR)-1,2,3,4,4a,5,6,7,8,8a-decahydroquinoline
CAS
22160-37-8
化学式
C9H17N
mdl
——
分子量
139.241
InChiKey
POTIYWUALSJREP-DTWKUNHWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:(4aS)-trans-decahydroquinoline 在 sodium tetrahydroborate 、 碘苯二乙酸 作用下, 以 2,2,2-三氟乙醇 为溶剂, 反应 19.0h, 生成 1-azabicyclo[5.3.0]decane参考文献:名称:NH哌啶环收缩为吡咯烷的氧化重排方法摘要:我们开发了一种利用 PhI(OAc)2 氧化重排来收缩 N-H 哌啶环的方法。该反应形成亚胺离子中间体,该中间体被亲核试剂(例如NaBH4、H2O)有效捕获,产生相应的吡咯烷衍生物。此外,我们提出了一种由实验和密度泛函理论计算支持的离子机制。DOI:10.1002/adsc.202400484
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 <(1R)-(endo,anti)>-(+)-3-bromocamphor-8-sulfonic acid 作用下, 生成 (4aS)-trans-decahydroquinoline参考文献:名称:Veneziani, Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, 1913, vol. <5> 22 II, p. 155摘要:DOI:
文献信息
-
A General Protocol for the Broad-Spectrum Cross-Coupling of Nonactivated Sterically Hindered 1° and 2° Amines作者:Abir Khadra、Stanislas Mayer、David Mitchell、Michael J. Rodriguez、Michael G. OrganDOI:10.1021/acs.organomet.7b00490日期:2017.9.25While notable advances have been reported for the metal-catalyzed cross-coupling of bulky amines, to date no set of reported conditions has proven general for both hindered and unactivated primary and secondary amines. Examples that are reported with Pd catalysts invariably involve aggressive alkoxide bases in order to provide the necessary “push” required to couple these challenging substrates. Consequently
-
CORROSION INHIBITION申请人:SCHLUMBERGER TECHNOLOGY CORPORATION公开号:US20180312980A1公开(公告)日:2018-11-01A corrosion inhibiting compound with a general structure A-B or A-X-B for inhibition of corrosion of steel in acidic solution. A comprises a heterocyclic ring system having a plurality of cyclic Carbon atoms and at least one cyclic Nitrogen atom, wherein the at least one cyclic Nitrogen atom is neutral under neutral conditions and protonatable under acidic conditions. B comprises at least two unsaturated Carbon atoms. B may comprise a ring system or a polymerisable group.一种具有一般结构A-B或A-X-B的防腐蚀化合物,用于抑制钢材在酸性溶液中的腐蚀。A包括具有多个环状碳原子和至少一个环状氮原子的杂环系统,其中至少一个环状氮原子在中性条件下是中性的,在酸性条件下可以质子化。B包括至少两个不饱和碳原子。B可以包括一个环系统或一个可聚合基团。
-
Regulating the growth of plants and controlling weeds with申请人:Ciba-Geigy Corporation公开号:US03941579A1公开(公告)日:1976-03-02Substituted azabicycloalkanes of the formula I ##EQU1## wherein R represents an alkyl radical which contains 2 or 3 carbon atoms and is substituted by chlorine or a straight-chain or branched alkenyl radical containing 3 or 4 carbon atoms, R.sub.1 and R.sub.2 represent hydrogen or the one represents methyl and the other represents hydrogen, and n is the number 1 or 2, may be used for influencing the growth of plants, preferably for combating weeds in rice-cultures.
-
Ophthalmic Compositions for Treating Ocular Hypertension申请人:Doherty James B.公开号:US20090062280A1公开(公告)日:2009-03-05This invention relates to the use of potent potassium channel blockers or a formulation thereof in the treatment of glaucoma and other conditions which leads to elevated intraocular pressure in the eye of a patient. This invention also relates to the use of such compounds to provide a neuroprotective effect to the eye of mammalian species, particularly humans.本发明涉及在治疗青光眼和其他导致患者眼内压升高的疾病中使用强效钾通道阻滞剂或其配方。本发明还涉及使用这些化合物在哺乳动物,特别是人类的眼中提供神经保护作用。
-
Thiazoles as inhibitors of 11B-hydroxysteroid dehydrogenase申请人:Gillespie Paul公开号:US20070167622A1公开(公告)日:2007-07-19Provided herein are compounds of the formula (I): as well as pharmaceutically acceptable salts thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of diseases such as, for example, type II diabetes mellitus and metabolic syndrome.本文提供了公式(I)的化合物及其药用可接受盐,其中取代基如规范中所述。这些化合物及其含有的药物组合物,可用于治疗疾病,例如II型糖尿病和代谢综合征。
表征谱图
-
氢谱1HNMR
-
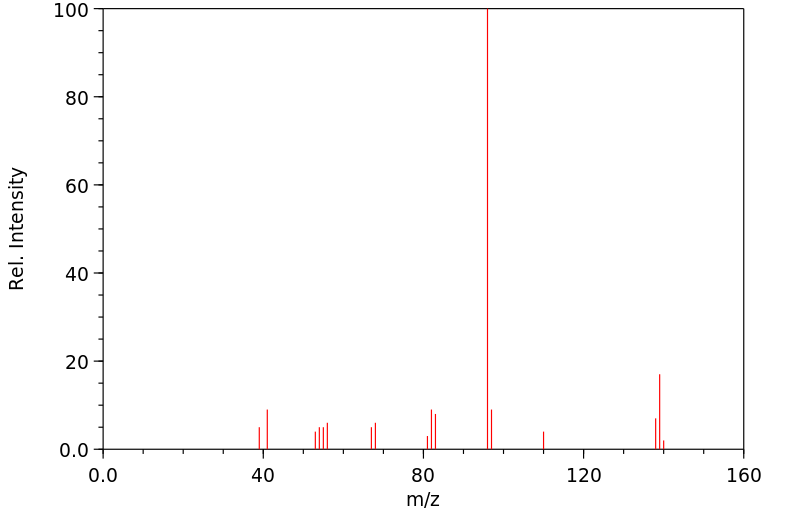
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锯齿石松宁
脱氧卡色素B
箭毒蛙毒素 C
环戊二烯并[b]吖庚英-5-醇,十氢-
坎库碘铵
十氢喹啉
十氢-2-甲基喹啉
八氢对苯二酚-4(1H)-酮
八氢喹啉-2(1H)-酮
八氢-2,6-喹啉二酮
二氯化硅2,3-萘醛菁
β-羟基丙基-环糊精
[(4aS,4bR,6aS,8S,10aS,10bS,12aS)-10a,12a-二甲基-1,2,3,4,4a,4b,5,6,6a,7,8,9,10,10b,11,12-十六氢萘并[6,5-f]喹啉-8-基]2-[4-[二(2-氯乙基)氨基]苯基]乙酸酯
[(4aS,4bR,6aS,8S,10aS,10bS,12aS)-1,10a,12a-三甲基-2-氧代-3,4,4a,4b,5,6,6a,7,8,9,10,10b,11,12-十四氢萘并[6,5-f]喹啉-8-基]2-[4-[二(2-氯乙基)氨基]苯基]乙酸酯
8H-13,3,6a-乙基亚基-7,10-亚甲基噁庚并[3,4-i]-1-苯并吖辛因-8-酮,1-乙基十四氢-12a-羟基-6-甲氧基-3-甲基-,(3R,6S,6aS,7R,7aS,10S,12aS,13S,13aR,15R)-(9CI)
8-羟基-十氢喹啉
4-乙炔基-2-甲基十氢喹啉-4-醇
4-乙炔基-2-甲基-1-(3-苯丙-2-炔-1-基)十氢喹啉-4-醇
3-羟基-13,17-开环-5-雄甾烯-17-酸-13,17-内酰胺(4-(二(2-氯乙基)氨基)苯基)丁酸酯
3-甲氧基-17-氮杂-高雄甾-5-烯-17-酮
2H-环戊二烯并[b]吡啶-2-酮,八氢-4-甲基-,[4S-(4-α-,4a-bta-,7a-bta-)]-(9CI)
2-甲基-1-(3-丙氧基-丙基)-八氢-喹啉-4-酮
2,5-二丙基十氢喹啉
1-(3-甲氧基-丙基)-2-甲基-八氢-喹啉-4-酮
1-(3-氯-丙基)-十氢-喹啉
1-(3-乙氧基-丙基)-2-甲基-十氢-喹啉
1-(3-乙氧基-丙基)-2-甲基-八氢-喹啉-4-酮
1,2,2-三甲基-八氢-喹啉-4-酮
(4aS,4bR,8S,10aR,10bS,12aS)-10a,12a-二甲基-2-羰基-1,2,3,4,4a,4b,5,7,8,9,10,10a,10b,11,12,12a-十六氢萘并[2,1-f]喹啉-8-基{4-[二(2-氯乙基)氨基]苯基}乙酸酯
(4aS,4bR,6aS,8S,10aS,10bS,12aS)-8-羟基-10a,12a-二甲基-3,4,4a,4b,5,6,6a,7,8,9,10,10b,11,12-十四氢-1H-萘并[2,1-f]喹啉-2-酮
(3S,13R)-1,2,3,4,4aalpha,5,11,11aalpha-八氢-2,2,5-三甲基-3beta,5beta-乙桥-10bH-吡啶并[3,2-b]咔唑-10bbeta,13-二醇
(3R,6S,6aS,7R,7aS,10S,12aS,13R,13aR,14S,15R)-1-乙基十四氢-12a,14-二羟基-6-甲氧基-3-甲基-8H-13,3,6a-亚乙基-7,10-甲桥氧杂卓并[3,4-i]-1-苯并氮杂环辛四烯-8-酮
(2S,4aR,8aR)-2-甲基八氢-4(1H)-喹啉酮
(2R,4R,4As,8As)-rel-4-乙炔基十氢-1,2-二甲基-4-喹啉醇
1-(2-Cyclopentylethyl)-perhydrochinolin
Perhydrodibenzochinolizin
octahydroquinoline-1(2H)-carbonitrile
N-Acetylbaikeidin
4-[4-[(4aR,8aR)-3,4,4a,5,6,7,8,8a-octahydro-2H-quinoline-1-carbonyl]thiophen-2-yl]piperidine-1-carboxamide
N-Chlor-trans-decahydrochinolin
(4aR,8aR)-1-{4-[4-(octahydro-quinoline-1(2H)-ylcarbonyl)-thiophen-2-yl]-piperidin-1-yl}-ethanone
(7-Acetyl-3a,6-dimethyl-3-oxo-tetradecahydro-7-aza-cyclohepta[e]inden-6-yl)-acetic acid
6,7-Cyclobutano-1,2-cyclopropano-chinolizidin
2-Methyl-2,3-tetramethylen-N-cyanoaziridin
(4aR)-2-oxo-1-[(1R)-1-phenylethyl]-4,5,6,7-tetrahydro-3H-quinoline-4a-carboxylic acid
(4aR,8aR)-(octahydro-quinolin-1(2H)-yl)-(5-piperidin-4-yl-thiophen-3-yl)-methanone
5-(octahydroquinolin-1-yl)-5-oxopentanoic acid N-benzyl-N-isopropylamide
3,4,4-Trimethyl-2-azabicyclo<3.3.0>octan
(4aR,8aR)-4-[4-(octahydro-quinoline-1(2H)-ylcarbonyl)-thiophen-2-yl]-piperidine-1-carboxylic acid tert-butyl ester
1-Aza-4,11-dioxo-3-oxo-methoxycarbonyl-tricyclo<5.3.1.05,10>undecan