赭曲霉毒素A | 303-47-9
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:28
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:113
-
氢给体数:3
-
氢受体数:6
ADMET
安全信息
-
储存条件:储存温度为2-8°C。
制备方法与用途
赭曲霉毒素A是一种典型的曲霉属真菌毒素,常见于谷物、咖啡、葡萄、葡萄酒和啤酒等中的次级代谢产物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— tert-butyl N-((5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-yl)carbonyl)-L-phenylalaninate —— C24H26ClNO6 459.927 1H-2-苯并吡喃-7-羧基LIC酸,5-氯-3,4-二氢-8-羟基-3-甲基-1-氧代 ochratoxin α 16281-39-3 C11H9ClO5 256.642 5-氯-8-羟基-3-甲基-1-氧代异色满-7-羧酸 ochratoxin α 19165-63-0 C11H9ClO5 256.642 —— ethyl 5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carboxylate 54870-23-4 C13H13ClO5 284.696 —— 7-ethoxycarbonyl-8-hydroxy-3-methyl-1-isochromanone 952023-80-2 C13H14O5 250.251 —— (R)-(-)-mellein methyl ether —— C11H12O3 192.214 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ochratoxin A —— C20H18ClNO6 403.819 N-[(5-氯-3,4-二氢-8-羟基-3-甲基-1-氧代-1H-2-苯并吡喃-7-基)羰基]-L-苯基丙氨酸甲酯 ochratoxin A methyl ester 16281-44-0 C21H20ClNO6 417.846 赭曲霉毒素 C ochratoxin C 4865-85-4 C22H22ClNO6 431.873 赭曲霉毒素 A-O-甲基,甲酯 ochratoxin A O-methyl ether methyl ester 4825-87-0 C22H22ClNO6 431.873 —— (4R)-hydroxyochratoxin A 35299-87-7 C20H18ClNO7 419.818 赭曲霉素 N-{[(3R)-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl]-carbonyl}-L-phenylalanine 4825-86-9 C20H19NO6 369.374 —— ochratoxin hydroquinone 205034-32-8 C20H19NO7 385.373 5-氯-8-羟基-3-甲基-1-氧代异色满-7-羧酸 ochratoxin α 19165-63-0 C11H9ClO5 256.642
反应信息
-
作为反应物:描述:赭曲霉毒素A 在 phosphate buffer 作用下, 以80%的产率得到ochratoxin hydroquinone参考文献:名称:Ochratoxin A acts as a photoactivatable DNA cleaving agent摘要:赭曲霉毒素A通过光诱导产生DNA断裂的能力被描述;在DNA存在的情况下,光反应产生非氯化的衍生物赭曲霉毒素B,而在无氧条件下则生成氢醌衍生物。DOI:10.1039/a708275d
-
作为产物:参考文献:名称:钌-NHC-二胺催化异香豆素的对映选择性氢化摘要:公开了一种新颖实用的手性钌-NHC-二胺体系,用于异香豆素的对映选择性氢化,为将(手性)NHC配体应用于不对称催化提供了一个新概念。以优异的对映选择性(高达 99% ee)获得了多种光学活性 3-取代的 3,4-二氢异香豆素。此外,该方法还用于合成 O-甲基蜜蜡、蜜蜡和赭曲霉毒素 A。DOI:10.1021/jacs.6b13124
文献信息
-
Structural and functional characterization of ochratoxinase, a novel mycotoxin-degrading enzyme作者:Doreen Dobritzsch、Huaming Wang、Gunter Schneider、Shukun YuDOI:10.1042/bj20140382日期:2014.9.15produced by Aspergillus and Penicillium species and occurs in a wide range of agricultural products. Detoxification of contaminated food is a challenging health issue. In the present paper we report the identification, characterization and crystal structure (at 2.2 Å) of a novel microbial ochratoxinase from Aspergillus niger. A putative amidase gene encoding a 480 amino acid polypeptide was cloned曲霉毒素以曲霉毒素A为主要形式,是对人类和动物最有害的五种主要霉菌毒素之一。它由曲霉和青霉菌种产生,并存在于多种农产品中。对受污染食品进行排毒是一个具有挑战性的健康问题。在本文中,我们报道了一种来自黑曲霉的新型微生物曲霉毒素酶的鉴定,表征和晶体结构(在2.2Å下)。克隆了一个假定的编码480个氨基酸多肽的酰胺酶基因,并在黑曲霉中同源表达。重组蛋白是N端截短的,热稳定的,在pH〜6和66°C下具有最佳活性,并且在ra曲霉毒素A水解中比羧肽酶A和Y(这两种已知的能够降解这种霉菌毒素的酶)更有效。同八聚体酶的亚基折叠成金属依赖性酰胺水解酶的两个结构域结构特征,具有扭曲的TIM(三磷酸磷酸异构酶)桶和较小的β-夹心结构域。该活性位点包含用于酸碱催化的天冬氨酸残基,以及用于结合双核金属中心的羧化赖氨酸残基和四个组氨酸残基。
-
Array Biosensor for Detection of Ochratoxin A in Cereals and Beverages作者:Miriam M. Ngundi、Lisa C. Shriver-Lake、Martin H. Moore、Michael E. Lassman、Frances S. Ligler、Chris R. TaittDOI:10.1021/ac048957y日期:2005.1.1Contamination of food by mycotoxins occurs in minute quantities, and therefore, there is a need for a highly sensitive and selective device that can detect and quantify these organic toxins. We report the development of a rapid and highly sensitive array biosensor for the detection and quantitation of ochratoxin A (OTA). The array biosensor utilizes a competitive immunoassay format. Immobilized OTA derivatives compete with toxin in solution for binding to fluorescent anti-OTA antibody spiked into the sample. This competition is quantified by measuring the formation of the fluorescent immunocomplex on the waveguide surface. The fluorescent signal is inversely proportional to the concentration of OTA in the sample. Analyses for OTA in buffer and a variety of food and beverage samples were performed. Samples were extracted with methanol, without any sample cleanup or preconcentration step prior to analysis. The limit of detection for OTA in several cereals ranged from 3.8 to 100 ng/g, while in coffee and wine, detection limits were 7 and 38 ng/g, respectively.
-
Ochratoxin A Forms a Carbon-Bonded C8-Deoxyguanosine Nucleoside Adduct: Implications for C8 Reactivity by a Phenolic Radical作者:Jian Dai、Marcus W. Wright、Richard A. MandervilleDOI:10.1021/ja034221r日期:2003.4.1oxidation to yield phenoxyl radicals. The C8 position of dG is susceptible to radical attack, as was amply proven through formation of the hydroxyl radical-derived DNA lesion, 8-oxodeoxyguanosine. The adduct 4 is the first structurally characterized nucleoside adduct of a chlorophenolic toxin, and its formation has important implications for the mutagenicity of phenolic xenobiotics.已使用电喷雾质谱和核磁共振评估了致癌真菌毒素赭曲霉毒素 A (OTA, 1) 与脱氧鸟苷 (dG) 反应的能力。在 50 mol 当量 dG 存在下对 OTA (100 muM) 进行光激发,导致分离和鉴定 C8-脱氧鸟苷核苷加合物 4。重要的是,使用辣根过氧化物酶 (HRP)/ OTA 氧化活化后形成了相同的加合物H2O2 或过渡金属 Fe(II) 和 Cu(II),如质谱所证实。因为人们认为 OTA 的致突变性和随后的致癌性源于氧化性 DNA 损伤(链断裂和氧化碱基产物)和鸟嘌呤特异性 DNA 加合物的形成,加合物 4 证实了 OTA 与 dG 共价反应的能力,并且对 OTA 和其他经过氧化产生苯氧基自由基的氯酚毒素的作用机制具有重要意义。dG 的 C8 位置易受自由基攻击,这一点已通过羟基自由基衍生的 DNA 损伤 8-氧代脱氧鸟苷的形成得到充分证明。加合物 4 是第一个具有结构特征
-
Metabolism of Ochratoxin A: Absence of Formation of Genotoxic Derivatives by Human and Rat Enzymes作者:Jean-Charles Gautier、Janique Richoz、Dieter H. Welti、Jovanka Markovic、Eric Gremaud、F. Peter Guengerich、Robert J. TureskyDOI:10.1021/tx000070j日期:2001.1.1and OTA products were not formed with horseradish peroxidase. There was no evidence of DNA adduct formation when [(3)H]OTA was incubated with these enzyme systems in the presence of calf thymus DNA (<20 adducts/10(9) DNA bases); however, these enzymes catalyzed DNA adduct formation with the genotoxins aflatoxin B(1), 2-amino-3-methylimidazo[4,5-f]quinoline, benzo[a]pyrene, and pentachlorophenol. Therech曲毒素A(OTA)在雄性大鼠中是一种强效的肾脏致癌物,尽管其致癌性模式尚不清楚。用细胞色素P450,谷胱甘肽S-转移酶,前列腺素H-合酶和辣根过氧化物酶体外研究了OTA与DNA的代谢和共价结合。将OTA与用NADPH强化的大鼠或人肝微粒体一起孵育可导致低速率[10-25 pmol min(-1)(mg蛋白质)(-1)]形成4-(R)-羟基och曲霉毒素A。没有证据表明大鼠,小鼠或人肾微粒体或线粒体后上清液(S-9)的OTA代谢和谷胱甘肽共轭物形成[<5 pmol min(-1)(mg蛋白)(-1)]。重组人细胞色素P450(P450)1A1和3A4以低比率形成[4-(R)-羟基p曲霉毒素A [分别为0.08和0.06 pmol min(-1)(pmol P450)(-1)];用重组人P450 1A2或2E1或大鼠P450 1A2或2C11未检测到OTA的氧化产物[<0.02 pmol min(-1)(pmol
-
On the role of copper and iron in DNA cleavage by ochratoxin A. Structure-activity relationships in metal binding and copper-mediated DNA cleavage作者:Jason A Ardus、Ivan G Gillman、Richard A MandervilleDOI:10.1139/v98-088日期:1998.6.1
Ochratoxin A (OTA, 1: X = Cl) is a fungal carcinogen that facilitates single-strand DNA cleavage and DNA adduction when metabolically activated. To determine if redox-active transition metals induce OTA-mediated DNA damage, we have examined the toxin's ability to bind Cu(II) and Fe(III) in aqueous media and facilitate DNA cleavage in their presence using agarose gel electrophoresis and supercoiled plasmid DNA. Using fluorescence spectroscopy, 1 was found to bind Cu(II) readily at physiological pH, while acidic conditions (pH 2.6) were employed to study Fe(III) binding due to the formation of Fe-oxide precipitates at higher pH values. Structure-activity relationships employing synthetic derivatives of 1 implied that 1 binds both Cu(II) and Fe(III) by its phenolic oxygen, while the carboxylic acid of its phenylalanine moiety binds Cu(II), but does not appear to play a role in Fe(III) coordination at pH 2.6. In terms of metal-mediated DNA cleavage, no role for 1 could be detected in Fe-induced DNA strand scission. With Cu(II), DNA cleavage by the 1:1 copper-bound complex of 1 could only be initiated by addition of a suitable reducing agent (sodium ascorbate). However, 1 was found to facilitate DNA cleavage by the Cu(II) complex of 1,10-phenanthroline (Cu(OP)2); a prototypical Cu-mediated nuclease system that cleaves DNA upon activation by an external reducing agent. Structure-activity relationships employing analogs lacking the chlorine atom, ochratoxin B (2: X = H), and the lactone (12), indicated that the chlorine atom is essential for activity of the OTA in potentiating DNA cleavage by Cu(OP)2. The implications of our findings to the genotoxic properties of 1 are discussed.Key words: ochratoxin, DNA cleavage, copper, iron, 1,10-phenanthroline.
奥克拉毒素A(OTA,1:X = Cl)是一种真菌致癌物质,当在代谢活化时,它促进单链DNA断裂和DNA加合物的形成。为了确定氧化还原活性过渡金属是否诱导OTA介导的DNA损伤,我们研究了毒素在水介质中结合Cu(II)和Fe(III)的能力,并在它们的存在下利用琼脂糖凝胶电泳和超螺旋质粒DNA促进DNA断裂。利用荧光光谱,发现1在生理pH下能够很容易地结合Cu(II),而在酸性条件(pH 2.6)下用于研究Fe(III)结合,因为在较高pH值下会形成Fe-氧化物沉淀。利用1的合成衍生物进行结构活性关系研究表明,1通过其酚氧结合Cu(II)和Fe(III),而其苯丙氨酸部分的羧酸结合Cu(II),但在pH 2.6下似乎不参与Fe(III)的配位。在金属介导的DNA断裂方面,未发现1在Fe诱导的DNA链切断中起作用。对于Cu(II),只有通过添加适当的还原剂(抗坏血酸钠)才能启动1的1:1铜结合复合物引发的DNA断裂。然而,发现1能够通过1,10-邻菲啰啉(Cu(OP)2)的Cu(II)复合物促进DNA断裂;这是一种原型的Cu介导核酸酶系统,只有在外部还原剂的激活下才能切割DNA。通过缺乏氯原子的类似物进行结构活性关系研究,如奥克拉毒素B(2:X = H)和内酯(12),表明氯原子对于OTA在促进Cu(OP)2介导的DNA断裂中的活性是必不可少的。我们的研究结果对于1的遗传毒性特性的影响进行了讨论。关键词:奥克拉毒素,DNA断裂,铜,铁,1,10-邻菲啰啉。
表征谱图
-
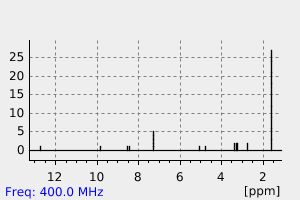
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息