4,5-二氢-2-十七烷基-1H-咪唑 | 105-28-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:94-95 °C
-
沸点:438.92°C (rough estimate)
-
密度:0.9055 (rough estimate)
-
保留指数:2647.8
计算性质
-
辛醇/水分配系数(LogP):7.6
-
重原子数:22
-
可旋转键数:16
-
环数:1.0
-
sp3杂化的碳原子比例:0.95
-
拓扑面积:24.4
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2933290090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-十七烷基-1,4,5,6-四氢嘧啶 2-heptadecyl-1,4,5,6-tetrahydropyrimidine 88097-26-1 C21H42N2 322.578 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-十七烷基-1,4,5,6-四氢嘧啶 2-heptadecyl-1,4,5,6-tetrahydropyrimidine 88097-26-1 C21H42N2 322.578 —— 2-heptadecyl-5-methyl-4,5-dihydro-1H-imidazole 121816-74-8 C21H42N2 322.578 —— 1-<2-Cyan-aethyl>-2-heptadecyl-4,5-dihydro-1H-imidazol 27243-34-1 C23H43N3 361.615
反应信息
-
作为反应物:描述:4,5-二氢-2-十七烷基-1H-咪唑 在 radium nickel 作用下, 以 正己烷 为溶剂, 以85 %的产率得到2-十七烷基咪唑参考文献:名称:一种2-十七烷基咪唑及其合成方法摘要:本发明属于表面活性剂领域,具体公开了一种2‑十七烷基咪唑及其合成方法,其主要合成路线如下:采用十八酸与乙二胺先合成十七烷基咪唑啉,再通过镭林镍催化脱氢生成2‑十七烷基咪唑。本发明产品在粉末涂料中有突出的应用效果,通过将咪唑链的链长短改为2‑十七烷基,较长的侧链降低了咪唑的活性使做成的粉末存储性能更加的优异,较长的碳链也增加了它的柔韧性,因此在高温烘烤时机械性能更佳,同时耐黄变性能也更加的突出;与此同时,较低的熔点也增加其与环氧树脂的相容性,在低温烘烤的时候同样有较好的流平的效果。除粉末涂料领域,此产品在胶粘剂、层压材料、涂料、电子油墨等领域也有突出的应用效果。公开号:CN116253684A
-
作为产物:描述:参考文献:名称:Butler, Richard N.; Thornton, John D.; O'Regan, C.B., Journal of the Chemical Society. Perkin transactions I, 1983, p. 2197 - 2200摘要:DOI:
文献信息
-
[EN] IMPROVEMENTS IN OR RELATING TO CURATIVES<br/>[FR] AMÉLIORATIONS APPORTÉES OU SE RAPPORTANT À DES AGENTS DE DURCISSEMENT申请人:HEXCEL COMPOSITES LTD公开号:WO2020216688A1公开(公告)日:2020-10-29The host compound in a clathrate is an amino or hydroxyl containing aromatic phosphorous compound, clathrates containing a resin curative and their use in curable resin compositions to produce moulded articles particularly fibre reinforced articles.
-
Process of preparing imidazolines申请人:IG FARBENINDUSTRIE AG公开号:US02176843A1公开(公告)日:1939-10-17
Imidazolines substituted in the 2-position are obtained by condensing N : N1-ethyleneurea, or an N : N1-ethyleneurea substituted at a carbon atom by a hydrocarbon radicle, with a monocarboxylic acid other than formic acid, the reaction occurring with elimination of water and carbon dioxide. In a typical example, N : N1-ethyleneurea and oleic acid, heated to 280-300 DEG C., yield 2-heptadecenyl-imidazoline. Examples are given also of the manufacture of imidazolines from N : N1-ethyleneurea and stearic acid, palmitic acid, benzoic acid, butyric acid, a - and b -naphthoic acids, diphenyl-4-carboxylic acid, carbazole-2-carboxylic acid, and an acid (molecular weight 238) obtained by the oxidation of paraffin. In a further example, N : N1-propylene urea is heated with benzoic acid to yield a mixture of 4- and 5-methyl-2-phenylimidazolines. Carbazole-2-carboxylic acid is obtainable by fusing 2-chloracetyl-N-acetylcarbazole with caustic potash.
-
Process of preparation of heterocyclic compounds employing acetylene ethers申请人:ORGANON公开号:US02813862A1公开(公告)日:1957-11-19
The invention comprises compounds of the formula <;FORM:0785373/IV (a)/1>; wherein R is a -(CH2)6- or <;FORM:0785373/IV (a)/2>; group and R2 is hydrogen, alkyl, aryl or aralkyl. It comprises also a process for the preparation of compounds of the formula <;FORM:0785373/IV (a)/3>; wherein X is O, S, NH or NR3 (R3 is an alkyl, aryl or aralkyl group), by reacting a compound of the formula R1OCC-R2, wherein R1 is an alkyl group, with a compound of the formula H2N-R-XH. The reaction is suitably carried out by allowing an alkoxyacetylene, such as an ethoxyacetylene compound, to react with an aminoalcohol, a diamine or a mercaptoamine. Examples describe the preparation according to the above process of (1) 2-methyl-oxazoline; (2) 2 : 4-dimethyl-oxazoline; (3) 2-methylimidazoline; (4) 2-methylbenzimidazole; (5) 2-methylthiazoline; (6) 2-methyl-1-dodecylimidazoline-2; (7) 2-propyl-imidazoline-2; (8) 2-benzylimidazoline-2; (9) 2-methyl- D 2-dihydro-oxazine - 1 : 3; (10) 2 - methyl - 3 : 4 : 5 : 6 - tetrahydropyrimidine; (11) 2-methyl-4 : 5-benzooxazine-1 : 3; (12) 2-methyl-5 : 6-dihydro-1 : 3-thiazine; (13) 2-(1-naphthylmethyl)-2-imidazoline; (14) 2-heptadecyl-2-imidazoline; (15) 2-methyl - 4 : 5 : 6 : 7 - tetrahydro - 1 : 3 - diazepine and (16) 2-methyl-4 : 5 : 6 : 7 : 8 : 9-hexahydro-1 : 3-oxazonine.
这项发明包括以下公式的化合物:<;FORM:0785373/IV (a)/1>;其中R是一个-(CH2)6-或<;FORM:0785373/IV (a)/2>;基团,R2是氢、烷基、芳基或芳基烷基。它还包括一种制备以下公式的化合物的方法:<;FORM:0785373/IV (a)/3>;其中X是O、S、NH或NR3(R3是烷基、芳基或芳基烷基),通过将公式R1OCC-R2的化合物(其中R1是烷基)与公式H2N-R-XH的化合物反应。反应通常通过允许烷氧基乙炔,如乙氧基乙炔化合物,与氨醇、二胺或巯胺反应来进行。示例描述了根据上述过程制备的化合物(1)2-甲基噁唑烷;(2)2 : 4-二甲基噁唑烷;(3)2-甲基咪唑烷;(4)2-甲基苯并咪唑;(5)2-甲基噻唑烷;(6)2-甲基-1-十二烷基咪唑-2;(7)2-丙基咪唑-2;(8)2-苄基咪唑-2;(9)2-甲基- D 2-二氢噁啉 - 1 : 3;(10)2-甲基-3 : 4 : 5 : 6-四氢嘧啶;(11)2-甲基-4 : 5-苯噁啉-1 : 3;(12)2-甲基-5 : 6-二氢-1 : 3-噻嗪;(13)2-(1-萘甲基)-2-咪唑烷;(14)2-庚十七烷基-2-咪唑烷;(15)2-甲基-4 : 5 : 6 : 7-四氢-1 : 3-二氮杂环己烷和(16)2-甲基-4 : 5 : 6 : 7 : 8 : 9-六氢-1 : 3-噁唑烷。 -
[EN] MULTINUCLEAR TRANSITION METAL POLYMERIZATION CATALYSTS<br/>[FR] CATALYSEURS DE POLYMERISATION DE METAL DE TRANSITION MULTINUCLEAIRE申请人:DOW CHEMICAL CO公开号:WO2004060901A1公开(公告)日:2004-07-22Multinuclear group 4 metal complexes useful as catalyst components for addition polymerizations and polymerization process, especially suited for preparing syndiotactic polymers of vinylaromatic monomers.
-
Catalyst activators comprising expanded anions申请人:——公开号:US20010027161A1公开(公告)日:2001-10-04A compound useful as a catalyst activator corresponding to the formula: (A* +a ) b (Z*J* j ) −c d , wherein: A* is a cation of charge +a, Z* is an anion group of from 1 to 50 atoms not counting hydrogen atoms, further containing two or more Lewis base sites; J* independently each occurrence is a Lewis acid coordinated to at least one Lewis base site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality, j is a number from 2 to 12 and a, b, c, and d are integers from 1 to 3, with the proviso that a×b is equal to c×d.一种有用的催化剂活化剂化合物,其化学式为:(A*+a)b(Z*J*j)−cd,其中:A*是带有+a电荷的阳离子,Z*是一个含有1至50个原子(不包括氢原子)的阴离子基团,进一步包含两个或更多的Lewis碱基位点;J*是独立的,每次出现时都是与Z*的至少一个Lewis碱基位点配位的Lewis酸,而且可选地,两个或更多这样的J*基团可以连接在一起,形成具有多个Lewis酸性功能的基团,j是从2到12的数字,a、b、c和d是从1到3的整数,但是a×b等于c×d。
表征谱图
-
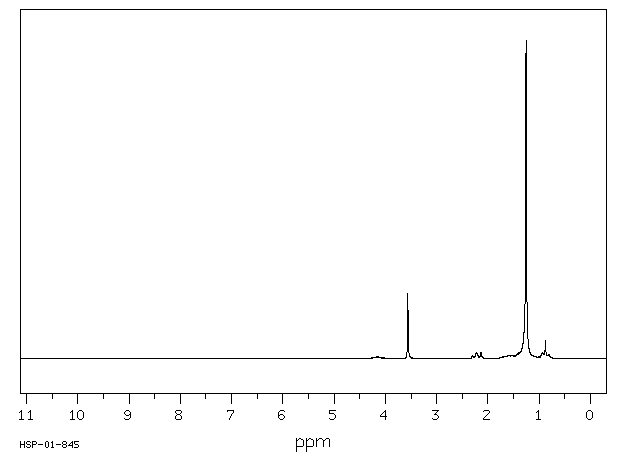
氢谱1HNMR
-
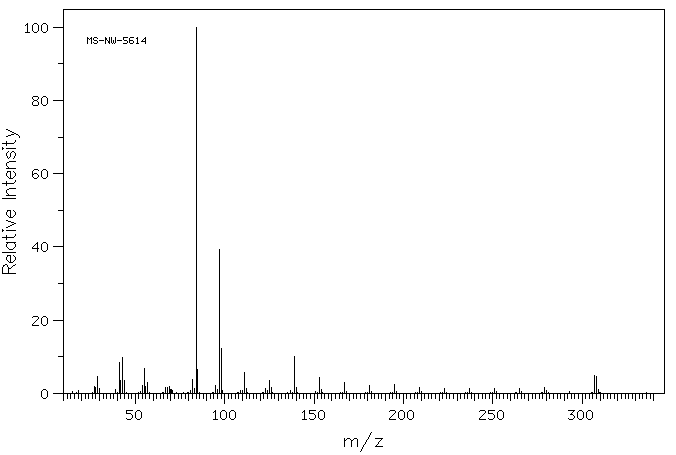
质谱MS
-
碳谱13CNMR
-
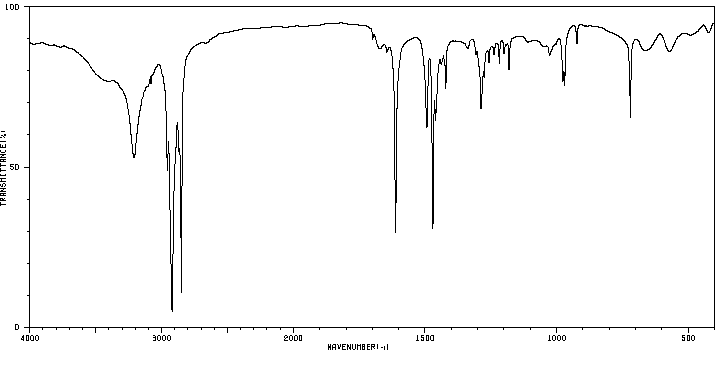
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息