二氧化钼 | 18868-43-4
中文名称
二氧化钼
中文别名
氧化钼;二氧化钼(IV)
英文名称
Molybdenum dioxide
英文别名
dioxomolybdenum
CAS
18868-43-4
化学式
MoO2
mdl
——
分子量
127.95
InChiKey
QXYJCZRRLLQGCR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:decomposes [JAN85]
-
密度:6.47 g/mL at 25 °C (lit.)
-
溶解度:不溶于水、酸性溶液、碱性溶液
-
暴露限值:a/nm
-
物理描述:DryPowder
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-0.24
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R20/21/22
-
WGK Germany:1
-
海关编码:2825700000
-
RTECS号:QA4688000
SDS
Section 1: Product Identification
Chemical Name: Molybdenum (IV) oxide, 99%
CAS Registry Number: 18868-43-4
Formula: MoO2
EINECS Number: 242-637-7
Chemical Family: metal oxide
Synonym: Molybdenum dioxide, Molybdenous oxide.
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 18868-43-4 100% 10mg/m3 (as Mo- 15mg/m3
Section 3: Hazards Identification
Emergency Overview: May be irritating to skin and eyes and respiratory tract.
Primary Routes of Exposure: Ingestion, inhalation
Eye Contact: May be a mild to severe irritant to the eyes.
Skin Contact: May cause slight to mild irritation of the skin.
Inhalation: Do not breath dust. Inhalation of powder may lead to irritation of the respiratory tract.
Ingestion: Ingestion may lead to vomiting and diarrhea.
Acute Health Affects: May be irritating to skin, eyes, mucous membranes and respiratory tract. Do not breath dust.
Chronic Health Affects: No information available on long-term chronic effects.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: not applicable
Autoignition Temperature: none
Explosion Limits: none
Extinguishing Medium: None. Material is non-flammable.
Special Fire Fighting Procedures: Non-flammable. No special fire fighting procedures.
Hazardous Combustion and No hazardous combustion or decomposition products.
Decomposion Products:
Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards.
SECTION 6: Accidental Release Measures
Spill and Leak Procedures: Small spills can be mixed with vermiculite or sodium carbonate and swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store in a tightly sealed container.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear appropriate chemical resistant gloves and protective clothing.
Ventilation: Material may form a fine dust. If possible, handle the material in an efficient fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: Material may form a fine dust. If possible, handle the material in an efficient fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: purple pwdr.
Molecular Weight: 127.95
Melting Point: no data
Boiling Point: no data
Vapor Pressure: no data
Specific Gravity: 6.47
Odor: none
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: air and moisture stable solid
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: none
Incompatibility: none
Decomposition Products: none
SECTION 11: Toxicological Information
RTECS Data: Subcutaneous (mouse); LD50:318 mg/kg.
Carcinogenic Effects: No data available
Mutagenic Effects: No data available
Tetratogenic Effects: No data available
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to local, state and federal regulations.
SECTION 14: Transportation
Shipping Name (CFR): Non-hazardous
Hazard Class (CFR): NA
Additional Hazard Class (CFR): NA
Packaging Group (CFR): NA
UN ID Number (CFR): NA
Shipping Name (IATA): Non-hazardous
Hazard Class (IATA): NA
Additional Hazard Class (IATA): NA
Packaging Group (IATA): NA
UN ID Number (IATA): NA
SECTION 15: Regulatory Information
TSCA: Listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三氧化钼 molybdenum trioxide 1313-27-5 MoO3 143.938
反应信息
-
作为反应物:描述:参考文献:名称:PULHAM, R. J.;RICHARDS, M. W.;EDWARDS, J., MATER. NUCL. REACTOR CORE APPL.: PROC. INT. CONF., BRISTOL, 27-29 OCT., 1+摘要:DOI:
-
作为产物:参考文献:名称:ZEKEL, ZH. A.;KRASNOBAEVA, N. B.;SHPIRT, M. YA., BCEC. SIMP. ZHROBL. KATAL. V UGLEXIMII, 21-27MAYA, 1990: TEZ. DOKL., DONE+摘要:DOI:
-
作为试剂:描述:3-甲基-2-丁烯醛 、 Magnesiumoxide 、 丙酮 在 二氧化钼 、 Zinc Oxide 作用下, 180.0 ℃ 、405.27 MPa 条件下, 反应 3.0h, 以27 Parts of 2-methyl-2,4-heptadien-6-one is obtained which的产率得到6-甲基-3,5-庚二烯-2-酮参考文献:名称:Production of .alpha.,.beta.-unsaturated ketones摘要:一种制备.alpha.,.beta.-不饱和酮的方法,包括在液相中存在锌氧化物催化剂的条件下,通过醛和酮的反应进行。所得的酮在某些情况下适用作溶剂,在某些情况下适用作有价值的香料、染料、塑料和特别是天然物质的中间体。公开号:US04005147A1
文献信息
-
BERG, LLOYD作者:BERG, LLOYDDOI:——日期:——
-
NOGITA, SHUNSUKE;MIYAMOTO, TOMOHIKO;KOYAMA, SHUNTARO, INT. CONF. COAL SCI., TOKYO, OCT. 23-27, 1989, SAN JOSE (CALIF.),(1989) C+作者:NOGITA, SHUNSUKE、MIYAMOTO, TOMOHIKO、KOYAMA, SHUNTARODOI:——日期:——
-
KATO, SIGEHRU;ROKUGAVA, NOBUNORI作者:KATO, SIGEHRU、ROKUGAVA, NOBUNORIDOI:——日期:——
-
HOFMANN, H.;MICHEL, B.;GAUCKLER, L. J.;ALLEMANN, J., CERAM. POWDER SCI. III: PROC. 3RD INT. CONF. POWDER PROCESS. SCI., SAN DI+作者:HOFMANN, H.、MICHEL, B.、GAUCKLER, L. J.、ALLEMANN, J.DOI:——日期:——
-
TSUTIYA, MITSURU作者:TSUTIYA, MITSURUDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
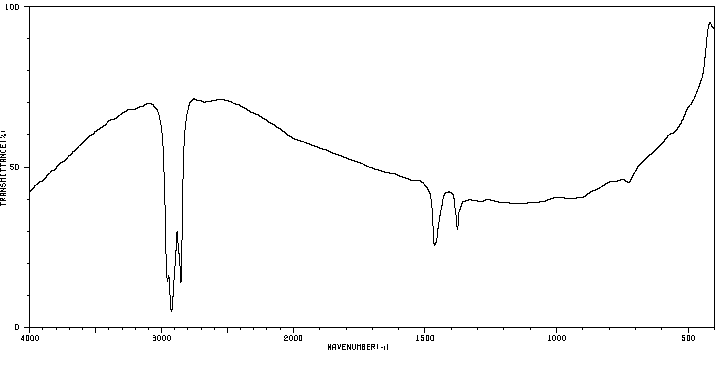
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息