氯磷酸二甲酯 | 813-77-4
中文名称
氯磷酸二甲酯
中文别名
O,O-二甲基磷酰氯;二甲基磷酰氯
英文名称
Dimethyl chlorophosphate
英文别名
phosphorochloridic acid dimethyl ester;dimethyl phosphorochloridate;[chloro(methoxy)phosphoryl]oxymethane
CAS
813-77-4
化学式
C2H6ClO3P
mdl
MFCD00117904
分子量
144.495
InChiKey
NGFFLHMFSINFGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:80 °C/25 mmHg (lit.)
-
密度:1.34 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:可溶于乙腈(少许)、氯仿(少许)
-
稳定性/保质期:
在常温常压下,该物质稳定。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:T+
-
安全说明:S26,S36/37/39,S45,S7/9
-
危险类别码:R26/27/28
-
WGK Germany:3
-
海关编码:29199000
-
危险品运输编号:UN 2927 6
-
储存条件:常温、避光、存于通风干燥处。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Dimethyl chlorophosphate
CAS-No. : 813-77-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Inhalation (Category 2)
Acute toxicity, Dermal (Category 2)
Acute toxicity, Oral (Category 2)
Skin corrosion (Category 1B)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Very toxic by inhalation, in contact with skin and if swallowed. Causes burns.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H300 Fatal if swallowed.
H310 Fatal in contact with skin.
H314 Causes severe skin burns and eye damage.
H330 Fatal if inhaled.
Precautionary statement(s)
P260 Do not breathe dust/ fume/ gas/ mist/ vapours/ spray.
P264 Wash hands thoroughly after handling.
P280 Wear protective gloves/ protective clothing/ eye protection/ face
protection.
P284 Wear respiratory protection.
P302 + P350 IF ON SKIN: Gently wash with plenty of soap and water.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R26/27/28 Very toxic by inhalation, in contact with skin and if swallowed.
R34 Causes burns.
S-phrase(s)
S 7/9 Keep container tightly closed and in a well-ventilated place.
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
S45 In case of accident or if you feel unwell, seek medical advice immediately
(show the label where possible).
Other hazards
Lachrymator.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : Dimethyl phosphorochloridate
Formula : C2H6ClO3P
Molecular Weight : 144,49 g/mol
Component Concentration
Dimethyl chlorophosphate
CAS-No. 813-77-4 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Take
victim immediately to hospital. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Oxides of phosphorus, Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid breathing vapors, mist or gas. Ensure adequate ventilation. Evacuate
personnel to safe areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling
the product.
Personal protective equipment
Eye/face protection
Tightly fitting safety goggles. Faceshield (8-inch minimum). Use equipment for eye protection
tested and approved under appropriate government standards such as NIOSH (US) or EN
166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: colourless
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 80 °C at 33 hPa - lit.
boiling range
g) Flash point 113 °C - closed cup
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,34 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong bases, Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be fatal if inhaled. Material is extremely destructive to the tissue of the
mucous membranes and upper respiratory tract.
Ingestion May be fatal if swallowed. Causes burns.
Skin May be fatal if absorbed through skin. Causes skin burns.
Eyes
Causes eye burns.
Signs and Symptoms of Exposure
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 2927 IMDG: 2927 IATA: 2927
UN proper shipping name
ADR/RID: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S. (Dimethyl chlorophosphate)
IMDG: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S. (Dimethyl chlorophosphate)
IATA: Toxic liquid, corrosive, organic, n.o.s. (Dimethyl chlorophosphate)
Transport hazard class(es)
ADR/RID: 6.1 (8) IMDG: 6.1 (8) IATA: 6.1 (8)
Packaging group
ADR/RID: I IMDG: I IATA: I
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 氯-二甲氧基膦 dimethyl chlorophosphite 3743-07-5 C2H6ClO2P 128.495
反应信息
-
作为反应物:参考文献:名称:Kir'yazov, M. P.; Belova, L. A.; Soifer, R. S., Journal of general chemistry of the USSR, 1987, vol. 57, # 6, p. 1130 - 1133摘要:DOI:
-
作为产物:参考文献:名称:磷酸、磺酸和羧酸的氯化物在 PTC 条件下在固体碳酸钾表面上的反应摘要:磷酸 (4) 和羧酸 (6) 酸酐的简单合成已经通过相转移催化酰化中的固体碳酸钾进行了阐述。在亲脂性季铵盐存在下,还研究了各种酰氯、磷酸 (1)、磺酸 (2) 和羧酸 (8) 对碳酸钾的行为。© 2004 Wiley Periodicals, Inc. 杂原子化学 15:447–450, 2004; 在线发表于 Wiley InterScience (www.interscience.wiley.com)。DOI 10.1002/hc.20039DOI:10.1002/hc.20039
-
作为试剂:描述:对硝基苯酚 、 S-(2-羟乙基)2,2-二甲基丙硫酸盐 在 三乙胺 、 氯磷酸二甲酯 作用下, 以 二氯甲烷 为溶剂, 反应 5.0h, 以50%的产率得到bis(S-pivaloyl-2-mercaptoethan-1-yl) (4-nitrophenyl) phosphate参考文献:名称:喹啉类化合物、其制备方法、药物组合物和用途摘要:一种如通式I所示的化合物、其药学上可接受的盐、或其代谢产物,以及其制备方法、药物组合物和用途。所示通式I所示的化合物、其药学上可接受的盐、或其代谢产物对病毒感染具有良好的治疗作用,毒副作用较小,可用于预防或治疗病毒感染。公开号:CN111777638A
文献信息
-
喹啉类衍生物及其制备方法与用途
-
[EN] FLAVIN DERIVATIVES<br/>[FR] DÉRIVÉS DE LA FLAVINE申请人:BIORELIX PHARMACEUTICALS INC公开号:WO2010019208A1公开(公告)日:2010-02-18The present invention relates novel flavin derivatives and other flavin derivatives, their use and compositions for use as riboswitch ligands and/or anti-infectives. The invention also provides method of making novel flavin derivatives.
-
Enantioselective Construction of Axially Chiral Amino Sulfide Vinyl Arenes by Chiral Sulfide‐Catalyzed Electrophilic Carbothiolation of Alkynes作者:Yaoyu Liang、Jieying Ji、Xiaoyan Zhang、Quanbin Jiang、Jie Luo、Xiaodan ZhaoDOI:10.1002/anie.201915470日期:2020.3.16The enantioselective construction of axially chiral compounds by electrophilic carbothiolation of alkynes is disclosed for the first time. This enantioselective transformation is enabled by the use of a Ts-protected bifunctional sulfide catalyst and Ms-protected ortho-alkynylaryl amines (Ts=tosyl; Ms=mesyl). Both electrophilic arylthiolating and electrophilic trifluoromethylthiolating reagents are
-
Synthesis and anti-diabetic activity evaluation of phosphonates containing thiazolidinedione moiety作者:Bogiri Sujatha、Subramanyam Chennamsetty、Venkataramaiah Chintha、Rajendra Wudayagiri、Kammela Prasada RaoDOI:10.1080/10426507.2020.1737061日期:2020.7.2−7.6 Kcal/mol) with the target gene, PPAR γ than the reference drug, Rosiglitazone (−7.4 Kcal/mol). In vitro anti-diabetic activity of the title compounds was also screened by standard α-amylase inhibition assay. Some of the tested compounds proved to possess promising activity when compared with the reference drug. Graphical Abstract摘要 以良好的收率合成了一系列含有噻唑烷二酮部分的取代膦酸酯。所有合成化合物的结构均通过 NMR(31P、1H 和 13C)和红外光谱、质谱和 C、H、N 元素分析证实。还进行了计算机分子对接研究,以评估它们在抗人 PPAR γ 蛋白配体上的相互作用和结合模式的抗糖尿病活性。从对接结果确定化合物(Z)-二甲基5-(3-硝基苯亚甲基)-2,4-二氧噻唑烷-3-基膦酸酯(7a)、(Z)-二甲基5-(3-氯-4 -氟苯亚甲基)-2,4-二氧噻唑烷-3-基膦酸酯 (7f), (Z)-二甲基 5-(2,4-二氯苯亚甲基)-2,4-二氧噻唑烷-3-基膦酸酯 (7e) 和 (Z)-二甲基5-((5-甲氧基吡啶-2-基)亚甲基)-2,4-dioxothiazolidin-3-ylphosphonate (7j) 与目标基因 PPAR γ 的结合能(-7.8、-7.6、-7.5 和 -7.6 Kcal/mol)比参考药物罗格列酮(-7
-
CHEMISTRY OF N-PHOSPHORYLATED NITROGEN MUSTARDS: THE EFFECT OF A SECOND NITROGEN SUBSTITUENT AT PHOSPHORUS ON THE STABILITY OF THE SYSTEM作者:Huijie Wan、Tomasz A. ModroDOI:10.1080/10426509608029648日期:1996.1.1Abstract Methyl N,N-diethyl-N'N'-bis(2-chloroethyl)phosphoramidate was prepared as a precursor for the corresponding phosphordiamidate anion, a model for the phosphoramidate mustard, biologically active degradation product of cyclophosphamide drug. Demethylation of the precursor led to a highly unstable ion which underwent spontaneous fragmentation. In the absence of an external nucleophile, the ion摘要 制备了 N,N-二乙基-N'N'-双(2-氯乙基)磷酰胺酸甲酯作为相应的二酰胺磷阴离子的前体,氨基磷酸芥的模型,环磷酰胺药物的生物活性降解产物。前体的去甲基化导致高度不稳定的离子发生自发分裂。在没有外部亲核试剂的情况下,离子分解产生偏氨基磷酸酯和 N-取代的乙烯亚胺作为主要中间体。在吡啶的存在下,两个吡啶发生双烷基化,产生双 [2-(N-吡啶并)乙基]胺二甲基化,此外还有一些 1,3,2-氧氮杂膦衍生物,通过竞争性 1,5-去甲基化阴离子的环化。在苯硫酚/三乙胺存在下孵育前体导致两个平行的亲核置换:(i) O-去甲基化,然后是两个苯硫酚分子的双烷基化,以及一些 1,5-环化;(ii) 初始直接分配...
表征谱图
-
氢谱1HNMR
-
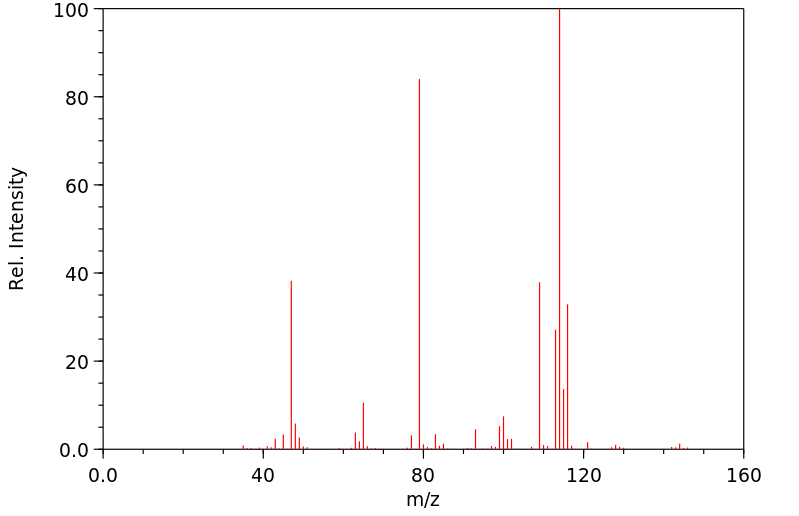
质谱MS
-
碳谱13CNMR
-
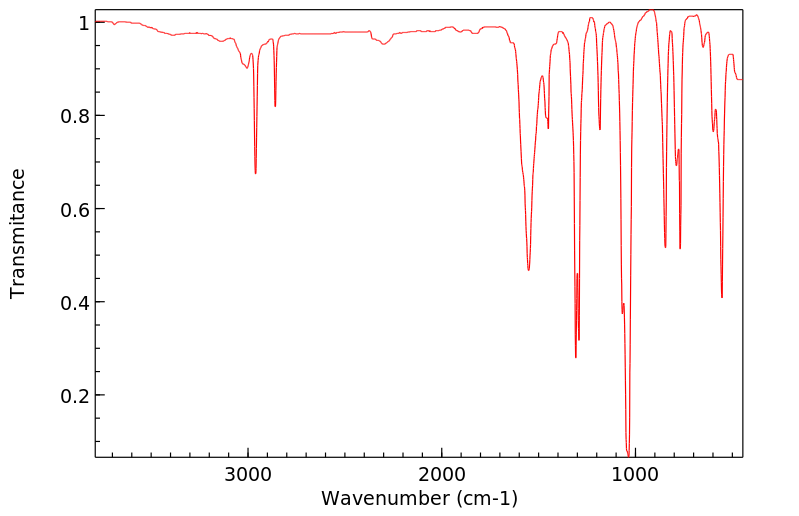
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(11bR,11''bR)-2,2''-[氧双(亚甲基)]双[4-羟基-4,4''-二氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11aR)-10,11,12,13-四氢-5-羟基-3,7-二-1-萘-5-氧化物-二茚基[7,1-de:1'',7''-fg][1,3,2]二氧杂磷杂八环
鲸蜡基磷酸-鲸蜡基磷酸二乙醇胺
高氯酸N,N,N',N',N'',N'',N''',N'''-八甲基二磷四酰胺(1:1:2)锂
非对称二乙基二(二甲基胺基)焦磷酸酯
非4-烯-5-基二苯基磷酸酯
雷公藤甲素O-甲基磷酸酯二苄酯
阿扎替派
间苯二酚双[二(2,6-二甲基苯基)磷酸酯]
锌四戊基二(磷酸酯)
银(1+)二苄基磷酸酯
铵4-(2-甲基-2-丁炔基)苯基4-(2-甲基-2-丙基)苯基磷酸酯
铵2-乙基己基磷酸氢酯
铵2,3-二溴丙基磷酸酯
钾二己基磷酸酯
钾二十烷基磷酸酯
钾二乙基磷酸酯
钾二(8-甲基壬基)磷酸酯
钾[5,7,7-三甲基-2-(1,3,3-三甲基丁基)辛基]磷酸酯
钾2-己基癸基磷酸酯
钴(2+)十三烷基磷酸酯
钡4,4-二乙氧基-2,3-二羟基丁基磷酸酯
钡1,3-二羟基-2-丙基磷酸酯
钠辛基氢磷酸酯
钠癸基氢磷酸酯
钠异丁基氢磷酸酯
钠二苄基磷酸酯
钠二戊基磷酸酯
钠二(十八烷基)磷酸酯
钠二(2-丁氧乙基)磷酸酯
钠O,O-二乙基磷酰蔷薇l烯酸酯
钠4-氨基苯基氢磷酸酯水合物(1:1:1)
钠3,6,9,12,15-五氧杂二十八碳-1-基氢磷酸酯
钠2-乙氧基乙基磷酸酯
钠2,3-二溴丙基磷酸酯
钛酸酯偶联剂NDZ-201
钙敌畏
钙二钠氟-二氧代-氧代膦烷碳酸盐
钙3,9-二氧代-2,4,8,10-四氧杂-3lambda5,9lambda5-二磷杂螺[5.5]十一烷3,9-二氧化物
野尻霉素6-磷酸酯
酸式磷酸戊酯
酚酞单磷酸酯
酚酞单磷酸环己胺盐
酚酞二磷酸四钠盐
酚酞二磷酸四钠
辛基磷酸酯
辛基二氯膦酸酯
辛基二氯丙基磷酸酯
辛基二丙基磷酸酯
赤藓糖醇4-磷酸酯