3-methyl-1,2-thiazinane 1,1-dioxide | 32446-08-5
中文名称
——
中文别名
——
英文名称
3-methyl-1,2-thiazinane 1,1-dioxide
英文别名
tetrahydro-3-methyl-2H-1,2-thiazine 1,1-dioxide;4-Methylbutansultam;3-Methylthiazinane 1,1-dioxide
CAS
32446-08-5
化学式
C5H11NO2S
mdl
——
分子量
149.214
InChiKey
YKURSMKKFYMPQJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:113 °C
-
沸点:241.7±23.0 °C(Predicted)
-
密度:1.156±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:54.6
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-tert.-Butyl-δ-chlor-pentansulfonamid 16867-23-5 C9H20ClNO2S 241.782
反应信息
-
作为产物:描述:1-戊烷磺酰胺 在 dirhodium tetraacetate 碘苯二乙酸 、 magnesium oxide 作用下, 以 二氯甲烷 为溶剂, 以70%的产率得到3-methyl-1,2-thiazinane 1,1-dioxide参考文献:名称:手性Dirhodium(II)配合物催化的磺酰胺类和磺胺类分子内不对称酰胺化反应摘要:脂族磺酰胺类化合物的对映选择性分子内酰胺化反应是首次通过手性羧甲基二铑(II)催化剂与PhI(OAc)2和MgO的结合实现,高收率且对映选择性高达66%(方案3,表1)。用[Rh 2 {((S)-nttl)4 ]和[Rh 2 {(R)-ntv)4 ]作为催化剂((S)-nttl =(αS)-α-(叔丁基)可获得最佳结果)-1,3-dioxo-2 H-苯并[ de ]异喹啉-2-乙酰基,(R)-nto =(αR)-α-异丙基-1,3-二氧杂-2- H-苯并[ de ]异喹啉-2-乙酰基)。此外,尽管这些羧酰二叉鎓(II)催化剂的分子内酰胺化反应也很有效,尽管这些后者反应的对映选择性明显较低(方案4,表3)。DOI:10.1002/hlca.200490148
文献信息
-
Phosphine Gold(I)-Catalyzed Hydroamination of Alkenes under Thermal and Microwave-Assisted Conditions作者:Xin-Yuan Liu、Cheng-Hui Li、Chi-Ming CheDOI:10.1021/ol060719x日期:2006.6.1[reaction: see text] Phosphine gold(I) complexes catalyzed isomerization of terminal alkenes and hydroamination of unactivated alkenes under thermal and microwave-assisted conditions. This is the first example of the use of microwave radiation as a heat source for gold(I)-catalyzed organic reactions.
-
Effective Intramolecular Hydrogen Abstraction by the Sulfonamide Radical作者:Takehisa Ohashi、Shinichi Takeda、Mitsuo Okahara、Saburo KomoriDOI:10.1246/bcsj.44.771日期:1971.3In benzene, the competitive hydrogen abstraction by the chlorine atom occurred to an appreciable extent and the participation of the chlorine atom was retarded with N2-flow control. In an aqueous solution (AcOH-H2O, t-BuOH-H2O), the quantitative conversion to γ-and δ-chloroalkanesulfonamide was observed; this was thought to be due to the intramolecular hydrogen abstraction by the sulfonamide radical
表征谱图
-
氢谱1HNMR
-
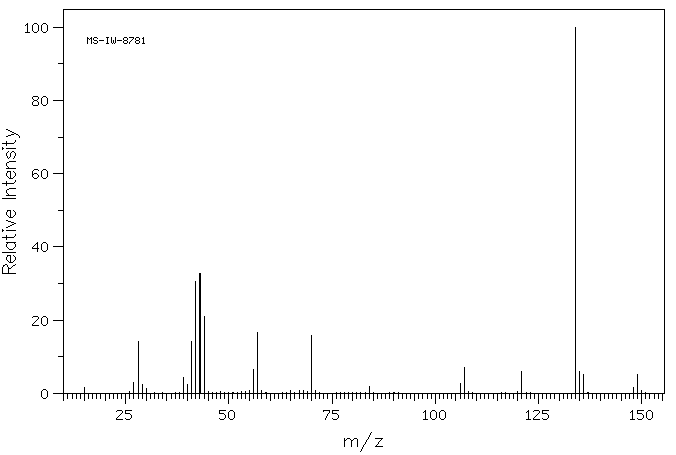
质谱MS
-
碳谱13CNMR
-
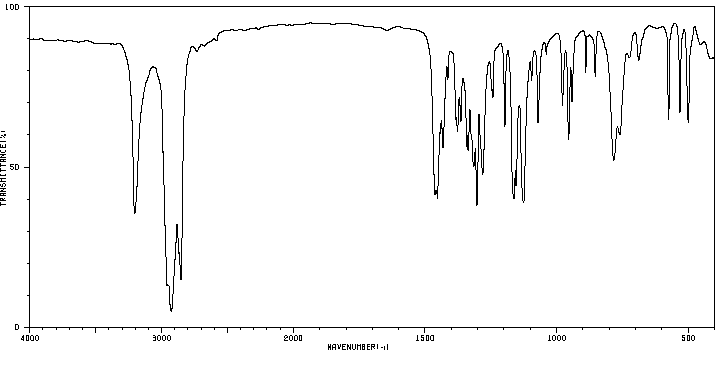
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3S,5S,6S)-安非他酮杂质
(3S,5R,6R)-安非他酮杂质
苯西酮
苯甲酸,4-(4-硫代吗啉基)-
硫代吗啉酮
硫代吗啉盐酸盐
硫代吗啉-4-醇1,1-二氧化物
硫代吗啉-4-甲酰氯-1,1-二氧化物
硫代吗啉-3-基甲醇
硫代吗啉-2-甲酸乙酯
硫代吗啉-1-鎓-1-醇
硫代吗啉-1,1-二氧化物
硫代吗啉,4-[4-[[2-(2,4-二氯苯基)-2-(1H-咪唑-1-基甲基)-1,3-二噁戊环-4-基]甲氧基]苯基]-,1-氧化,顺-(9CI)
硫代吗啉,3-乙基-2-甲基-
硫代吗啉 1,1-二氧化物盐酸盐
硫代吗啉
甲基2-乙氧基-6H-1,3-噻嗪-5-羧酸酯
甲基2-(甲基氨基)-4-氧代-5,6-二氢-4H-1,3-噻嗪-6-羧酸酯
甲基(2Z)-3-苄基-2-(苄基亚氨基)-4-氧代-1,3-噻嗪烷-6-羧酸酯
甲基(2Z)-3-异丙基-2-(异丙基亚胺)-4-氧代-1,3-噻嗪烷-6-羧酸酯
巯基吗啉-4-甲酸叔丁酯
四氢-3-甲基-2-苯基-4H-1,3-噻嗪-4-酮1,1-二氧化物
四氢-1,4-噻嗪-3,5-二酮
噻唑并[2,3-c][1,4]噻嗪-3(2H)-硫酮,8a-乙基四氢-8-甲基-
反式环戊烯三硫代碳酸酯
二苯甲基{5-[(4,6-二脱氧六吡喃糖基)氧代]-2,4,6-三羟基环己烷-1,3-二基}二(甲基氨基甲酸酯)
n-boc-2-硫代吗啉羧酸乙酯
[(2Z)-3-氰基-1,3-噻唑烷-2-亚基]氰胺
N-甲基四氢-1,2-噻嗪S,S-二氧化物
N-甲基-4-硫代吗啉甲酰胺
N-环己基-5,6-二氢-4H-1,3-噻嗪-2-胺
N-亚硝基硫代吗啉
N-丁基-5,6-二氢-4H-1,3-噻嗪-2-胺
N-Boc-1,4-噻嗪S,S-二氧化物
N-(3-氨基丙基)-硫代吗啉
N-(2-羟基丙基)硫代吗啉
N-(2-羟乙基)吗啉
AMT盐酸盐
6-苄基-2-甲基噻嗪1,1-二氧化物
6-羟基-5,6-二甲基-1,3-噻吖己环-2-硫酮
6-甲基-4-苯基硫代吗啉-3-酮
6-甲基-2-苯基-5,6-二氢-4H-1,3-噻嗪
6-甲基-1,3-噻嗪-2-硫酮
6-异丙基-3-硫代吗啉酮1-氧化物
6-异丙基-3-硫代吗啉酮
6-丙基-硫代吗啉-3-酮
6-(丁氧基甲基)-4-苯基硫代吗啉-3-酮
6,6-二甲基-1,4-噻嗪-2,5-二甲酸
5H-[1,3]噻唑并[5,4-h][1,4]苯并噻嗪
5-甲基-6-(吡啶-3-基)硫代吗啉-3-酮盐酸(1:1)