三異丙苯 | 717-74-8
中文名称
三異丙苯
中文别名
均三异丙基苯;三异丙基苯;1,3,5-三异丙基苯;1,3,5-三异丙苯;间三异丙苯
英文名称
1,3,5-triisopropyl benzene
英文别名
TIPB;triisopropylbenzene;1,3,5-tris(1-methylethyl)benzene;2,4,6-triisopropylbenzene;1,3,5-TIPB;1,3,5-Triisopropylbenzene;1,3,5-tri(propan-2-yl)benzene
CAS
717-74-8;27322-34-5
化学式
C15H24
mdl
MFCD00008890
分子量
204.356
InChiKey
VUMCUSHVMYIRMB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-14--11°C
-
沸点:232-236 °C (lit.)
-
密度:0.845 g/mL at 25 °C (lit.)
-
闪点:188 °F
-
LogP:6.68 at 24℃
-
物理描述:Liquid
-
保留指数:1322.6;1322.8;1324.3;1327;1325;1326;1327;1324.9;1325.7;1326.7;1327;1327;1322.6;1329
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S23,S24/25
-
WGK Germany:3
-
海关编码:29029080
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-二异丙基苯 1,3-diisopropylbenzene 99-62-7 C12H18 162.275 异丙苯 Isopropylbenzene 98-82-8 C9H12 120.194 1,3,5-三正丙基苯 1,3,5-tripropylbenzene 15181-14-3 C15H24 204.356 1,2,4-三异丙基苯 1,2,4-triisopropylbenzene 948-32-3 C15H24 204.356 1,2,4,5-四异丙苯 1,2,4,5-tetraisopropylbenzene 635-11-0 C18H30 246.436 1,4-二异丙基苯 1,4-bis(1-methylethyl)benzene 100-18-5 C12H18 162.275 alpha,alpha,alpha',alpha',alpha'',alpha''-六甲基苯-1,3,5-三甲醇 1,3,5-tri(2-hydroxyisopropyl)benzene 19576-38-6 C15H24O3 252.354 二异丙苯 1,2-diisopropylbenzene 577-55-9 C12H18 162.275 三乙酰基苯 1,3,5-triacetylbenzene 779-90-8 C12H12O3 204.225 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3-二异丙基苯 1,3-diisopropylbenzene 99-62-7 C12H18 162.275 异丙苯 Isopropylbenzene 98-82-8 C9H12 120.194 —— 1-(3,5-diisopropylphenyl)ethan-1-one —— C14H20O 204.312 3,5-双(1-甲基乙基)苯酚 3,5-diisopropylphenol 26886-05-5 C12H18O 178.274 1,2,4-三异丙基苯 1,2,4-triisopropylbenzene 948-32-3 C15H24 204.356 —— 2.4.6-Tri-i-propyltoluol 6319-85-3 C16H26 218.382 —— 2-(3,5-diisopropyl-phenyl)-propan-2-ol 19576-36-4 C15H24O 220.355 —— 1,3,5-Triisopropyl-2,4-dimethylbenzol 57440-46-7 C17H28 232.409 1,4-二异丙基苯 1,4-bis(1-methylethyl)benzene 100-18-5 C12H18 162.275 —— 1,3-bis-(α-hydroxy-isopropyl)-5-isopropyl-benzene 19576-37-5 C15H24O2 236.354 alpha,alpha,alpha',alpha',alpha'',alpha''-六甲基苯-1,3,5-三甲醇 1,3,5-tri(2-hydroxyisopropyl)benzene 19576-38-6 C15H24O3 252.354 —— 1,3,5-(2-chloroisopropyl)benzene 77367-66-9 C15H21Cl3 307.691 1,3,5-三(1-溴-1-甲基乙基)苯 1,3,5-Tris(1-bromisopropyl)benzol 41009-71-6 C15H21Br3 441.044 —— 1,2,4,5-Tetraisopropyl-benzol 29040-93-5 C18H30 246.436 2-氯-1,3,5-三异丙基苯 1-chloro-2,4,6-tri(iso-propyl)benzene 55538-62-0 C15H23Cl 238.801 二异丙苯 1,2-diisopropylbenzene 577-55-9 C12H18 162.275 —— 1-iodo-2,4,6-triisopropylbenzene 2100-22-3 C15H23I 330.252 三乙酰基苯 1,3,5-triacetylbenzene 779-90-8 C12H12O3 204.225 —— 2,4,6-triisopropylaniline 21524-36-7 C15H25N 219.37 2,4,6-三异丙基苯酚 2,4,6-triisopropylphenol 2934-07-8 C15H24O 220.355 - 1
- 2
反应信息
-
作为反应物:描述:三異丙苯 在 lithium aluminium tetrahydride 作用下, 生成 2.4.6-Tri-i-propyltoluol参考文献:名称:Nilsson,A.; Olsson,K., Acta chemica Scandinavica. Series B: Organic chemistry and biochemistry, 1975, vol. 29, p. 752 - 756摘要:DOI:
-
作为产物:参考文献:名称:Disproportionation of Alkylbenzenes. III. Spectral Characteristics and Other Physical Properties of Symmetrical Trialkylbenzenes. Proof of Structure of 1,3,5-Tri-t-butylbenzene1摘要:DOI:10.1021/ja01638a020
-
作为试剂:参考文献:名称:共价固定在连续流微反应器中的羟基腈裂解酶†摘要:当涉及高对映体纯度时,酶是最高的催化剂,酶的固定化将为连续操作铺平道路。在这种情况下,我们显示了在一个硅质整体式微反应器中,羟基腈裂解酶Hb HNL(来自巴西橡胶树)和Me HNL(来自Manihot esculenta)的共价固定化,以实现连续操作。在介孔硅酸盐上对固定化的HNL进行了彻底的表征,表明了成功进行固定化的必要条件。它们在连续流系统中的应用使得使用最少的酶负载量(STY = 71 g L -1 h -1)即可以极高的转化率(97%)和高ee(98%)快速生产(3.2分钟)手性氰醇毫克蛋白质-1)。Me HNL显示出增加的操作稳定性,可能是由于结构上的差异。连续流微反应器的性能优于分批系统,这证明了介孔/大孔环境在表达酶活性方面的优势以及微反应器的良好特性。总体而言,该系统在生物催化不对称合成的未来工业应用中显示出巨大的潜力。DOI:10.1039/c8cy02192a
文献信息
-
Versatile direct dehydrative approach for diaryliodonium(iii) salts in fluoroalcohol media作者:Toshifumi Dohi、Motoki Ito、Koji Morimoto、Yutaka Minamitsuji、Naoko Takenaga、Yasuyuki KitaDOI:10.1039/b708802g日期:——We have found that the use of fluoroalcohol media greatly enhanced the efficiency and scope of the direct dehydrative condensation of arenes1 and hypervalent iodine(III) compounds; the present clean method has a broad range of applicability as well as unique selectivity in the aromatic substrates, and is highly efficient even in polymer functionalization.
-
A Metal‐Free Direct Arene C−H Amination作者:Tao Wang、Marvin Hoffmann、Andreas Dreuw、Edina Hasagić、Chao Hu、Philipp M. Stein、Sina Witzel、Hongwei Shi、Yangyang Yang、Matthias Rudolph、Fabian Stuck、Frank Rominger、Marion Kerscher、Peter Comba、A. Stephen K. HashmiDOI:10.1002/adsc.202100236日期:2021.6.8The synthesis of aryl amines via the formation of a C−N bond is an essential tool for the preparation of functional materials, active pharmaceutical ingredients and bioactive products. Usually, this chemical connection is only possible by transition metal-catalyzed reactions, photochemistry or electrochemistry. Here, we report a metal-free arene C−H amination using hydroxylamine derivatives under benign
-
The palladium-catalyzed desulfitative cyanation of arenesulfonyl chlorides and sodium sulfinates作者:Jianbin Chen、Yang Sun、Bin Liu、Dongfang Liu、Jiang ChengDOI:10.1039/c1cc16134b日期:——A palladium-catalyzed desulfitative cyanation of arenesulfonyl chlorides and sodium sulfinates has been developed, providing aryl nitriles in moderate to excellent yields. It represents a facile procedure to access aryl nitriles.
-
PHOSPHORUS-CONTAINING CATALYSTS申请人:AGENCY FOR SCIENCE, TECHNOLOGY AND RESEARCH公开号:US20160207034A1公开(公告)日:2016-07-21The invention provides compounds of general structure I: (Ar 1 —Ar 2 —Ar 3 -E-P(=D)R 2 -) n M m X n L n ″. In this structure: •Ar 1 , Ar 2 and Ar 3 are aromatic groups wherein: —Ar 1 and Ar 3 are in a 1,3 relationship on Ar 2 , —each of Ar 1 , Ar 2 and Ar 3 optionally comprises one or more ring substituents of formula YR′ r wherein each Y independently is absent or is O, S, B, N or Si and each R′ is independently H, halogen, alkyl, cycloalkyl, aryl or heteroaryl and r is 1, 2 or 3, where r is 1 if Y is absent or is O or S, 2 if Y is B or N and 3 if Y is Si, —Ar 1 , Ar 2 and Ar 3 are each independently carbocyclic or heterocyclic and each is independently monocyclic, bicyclic or polycyclic and each ring of each of Ar 1 , Ar 2 and Ar 3 independently has 5, 6 or 7 ring atoms; •E is absent or is selected from the group consisting of O, S, NR″, SiR″ 2 , AsR″ 2 and CR″ 2 ; •M is a complexing metal; •X is selected from the group consisting of H, F, Br, CI, I, OTf, dba (dibenzylidene acetone), OC(═O)CF 3 and OAc; •L is selected from the group consisting of PR″ 2 , NR″ 2 , OR″, SR″, SiR″ 3 , AsR″ 3 , alkene, alkyne, aryl and heteroaryl, each of said alkene, alkyne, aryl and heteroaryl being optionally substituted, for example with one or more halogens and/or with one or more R groups as defined herein; •each R is independently alkyl, cycloalkyl, heterocyclyl, heterocycloalkyl, aryl or -, heteroaryl; •D is absent or is ═S or —O or —Z-linker-Z—, where each Z independently is O or NH or N-alkyl and linker is an alkyl chain of 2-5 carbon atoms in length; •each R″ is independently H, alkyl, cycloalkyl, heterocycloalkyl, aryl or heteroaryl, each other than H being optionally substituted, or R″ 2 is —Z-linker-Z— as defined above; and •m is 0 or 1 or 2; wherein if m is 0, n is 1, n′ and n″ are 0 and -- is absent; and if m is 1 or 2, n is 1 or 2 and n′ and n″ are integers such that the coordination sphere of M is filled, and D is absent.该发明提供了一般结构I的化合物:(Ar1—Ar2—Ar3-E-P(=D)R2-)nMmXnLn″。在这个结构中: •Ar1,Ar2和Ar3是芳香族基团,其中:—Ar1和Ar3在Ar2上呈1,3关系,—Ar1,Ar2和Ar3中的每一个可选地包括一个或多个环取代基,其化学式为YR′r,其中每个Y独立地不存在或为O、S、B、N或Si,每个R′独立地为H、卤素、烷基、环烷基、芳基或杂芳基,r为1、2或3,其中如果Y不存在或为O或S,则r为1,如果Y为B或N,则r为2,如果Y为Si,则r为3,—Ar1,Ar2和Ar3分别独立地为碳环或杂环,每个分别独立地为单环、双环或多环,每个Ar1,Ar2和Ar3的每个环独立地具有5、6或7个环原子; •E不存在或从O、S、NR″、SiR″2、AsR″2和CR″2组成的群体中选择; •M是络合金属; •X从H、F、Br、Cl、I、OTf、dba(双苯亚乙酮)、OC(═O)CF3和OAc组成的群体中选择; •L从PR″2、NR″2、OR″、SR″、SiR″3、AsR″3、烯烃、炔烃、芳基和杂芳基组成的群体中选择,所述的每个烯烃、炔烃、芳基和杂芳基可选地被取代,例如用一个或多个卤素和/或如本文所定义的一个或多个R基取代; •每个R独立地为烷基、环烷基、杂环烷基、杂环烷基、芳基或-、杂芳基; •D不存在或为═S或—O或—Z-连接-Z—,其中每个Z独立地为O或NH或N-烷基,连接体为长度为2-5个碳原子的烷基链; •每个R″独立地为H、烷基、环烷基、杂环烷基、芳基或杂芳基,除H外的每个均可选地被取代,或R″2为—Z-连接-Z—如上所定义;和 •m为0或1或2;其中如果m为0,n为1,n′和n″为0且--不存在;如果m为1或2,n为1或2,n′和n″为整数,使得M的配位球填满,并且D不存在。
-
Palladium Catalyzed Aryl(alkyl)thiolation of Unactivated Arenes作者:Perumal Saravanan、Pazhamalai AnbarasanDOI:10.1021/ol4036209日期:2014.2.7palladium-catalyzed aryl(alkyl)thiolation of various substituted unactivated arenes is accomplished for the synthesis of diverse unsymmetrical diaryl(alkyl) sulfides in good yield employing electrophilic sulfur reagent 6 derived from succinimide. The developed strategy was coupled with intramolecular arylation of a C–H bond to afford dibenzothiphene derivatives, an important moiety in material science as organic
表征谱图
-
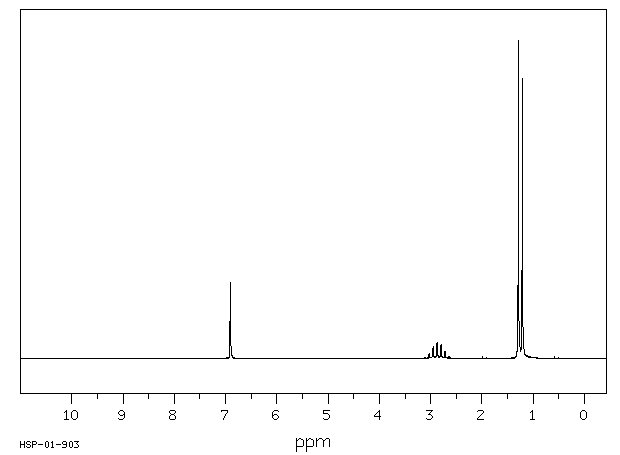
氢谱1HNMR
-
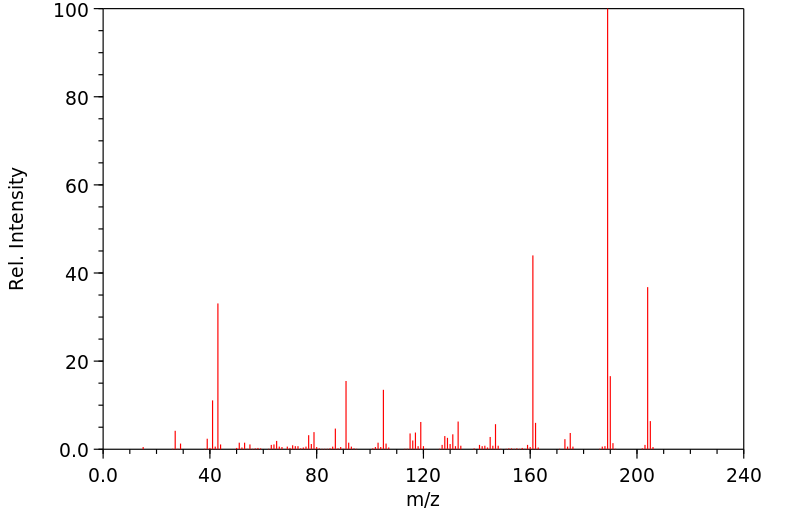
质谱MS
-
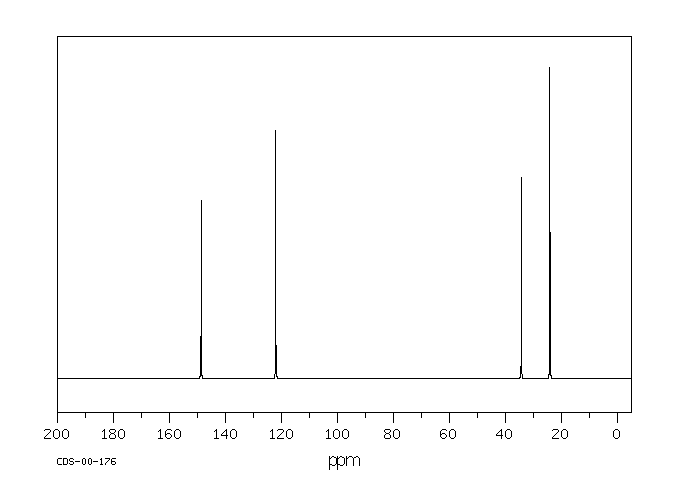
碳谱13CNMR
-
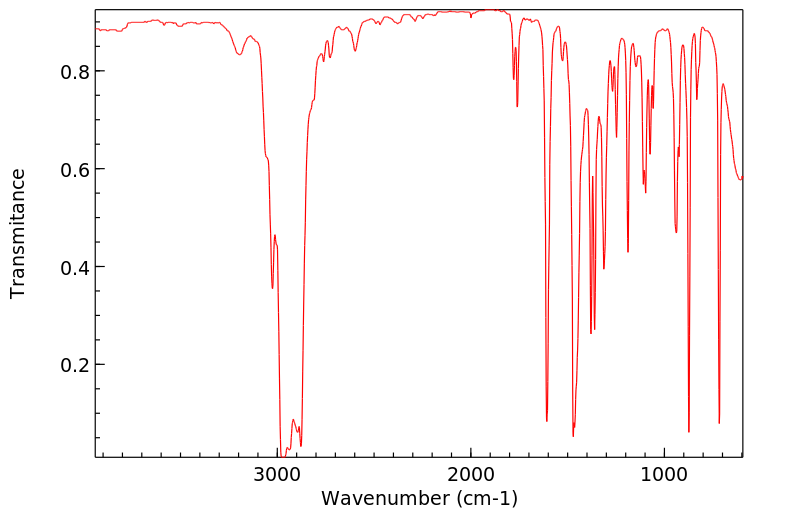
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫