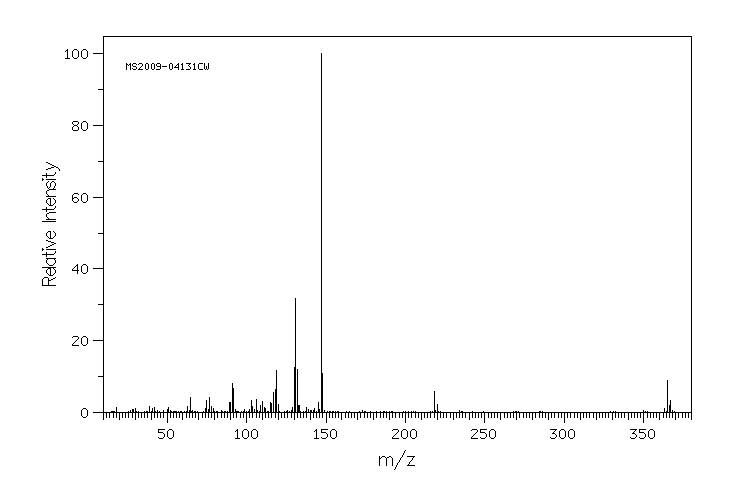
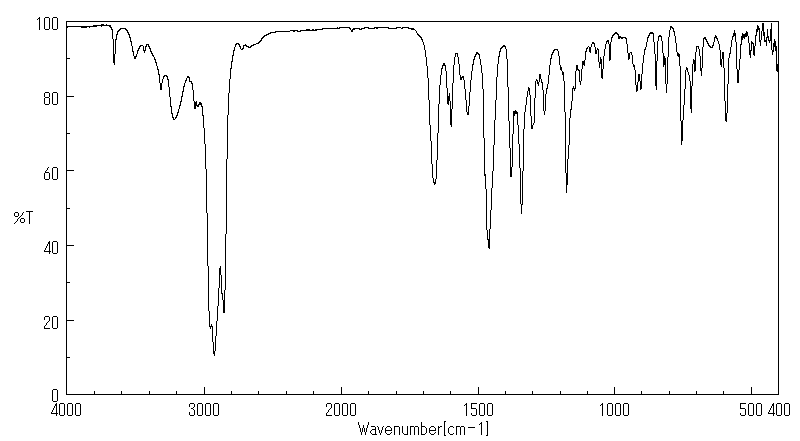
代谢
由于在肝脏内广泛代谢,大部分被排泄的吲达帕胺都已代谢,只有7%保持不变。在人体内,可能产生多达19种不同的吲达帕胺代谢物,尽管并非所有都已识别。吲达帕胺的代谢途径有几种,CYP3A4是参与相应羟基化、羧基化和脱氢反应的主要酶。吲达帕胺可以经过脱氢反应形成M5,然后氧化形成M4,再在吲哚部分进一步羟基化形成M2。这些反应由CYP3A4促进。另一种代谢途径是吲达帕胺首先被CYP3A4羟基化为M1。M1然后经过脱氢反应形成M3,并进一步氧化形成M2。根据动物研究,吲达帕胺的吲哚部分羟基化被认为是形成主要代谢物(M1),与母体化合物相比,其药理活性较低。吲达帕胺还可能通过CYP3A4发生环氧化反应,形成一个反应性环氧中间体。不稳定的环氧中间体然后可能通过微粒体环氧水合酶发生二羟基化形成M6,或者与谷胱甘肽结合形成M7。
As a result of extensive metabolism in the liver, the majority of indapamide excreted is metabolized, with only 7% remaining unchanged. In humans, as many as 19 distinct indapamide metabolites may be produced, although not all have been identified. There are several metabolic routes through which indapamide may be metabolized, and CYP3A4 is the main enzyme involved in the corresponding hydroxylation, carboxylation, and dehydrogenation reactions. Indapamide can undergo dehydrogenation to form M5, then oxidation to form M4, then further hydroxylation at the indole moiety to form M2. These reactions are facilitated by CYP3A4. Another route of metabolism occurs when indapamide is first hydroxylated to M1 by CYP3A4. M1 then undergoes dehydrogenation to form M3 and is further oxidized to form M2. Hydroxylation of indapamide’s indole moiety is thought to form the major metabolite (M1), which is less pharmacologically active compared to its parent compound according to animal studies. Indapamide may also undergo epoxidation via CYP3A4 to form a reactive epoxide intermediate. The unstable epoxide intermediate may then undergo dihydroxylation via microsomal epoxide hydrolase to form M6, or glutathione conjugation to form M7.
来源:DrugBank