| Name: | Carbon Tetrachloride, 99+%, Spectrophotometric Grade Material Safety Data Sheet |
| Synonym: | Tetrachloromethane; Carbon tet; Carbona; Carbon chloride; Methane tetrachloride. |
| CAS: | 56-23-5 |
Section 1 - Chemical Product MSDS Name: Carbon Tetrachloride, 99+%, Spectrophotometric Grade Material Safety Data Sheet
Synonym: Tetrachloromethane; Carbon tet; Carbona; Carbon chloride; Methane tetrachloride.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS | CAS# | Chemical Name | content | EINECS# |
| 56-23-5 | Carbon Tetrachloride | >99 | 200-262-8 |
Hazard Symbols: T N
Risk Phrases: 23/24/25 40 59 48/23 52/53
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Toxic by inhalation, in contact with skin and if swallowed. Limited evidence of a carcinogenic effect. Dangerous for the ozone layer.
Toxic : danger of serious damage to health by prolonged exposure through inhalation. Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment.Cancer suspect agent. Potential Health Effects
Eye:
Causes eye irritation. Vapors cause eye irritation.
Skin:
Causes skin irritation. May be absorbed through the skin in harmful amounts. Contact with the skin defats the skin.
Ingestion:
May cause liver and kidney damage. May cause central nervous system depression, characterized by excitement, followed by headache, dizziness, drowsiness, and nausea. Advanced stages may cause collapse, unconsciousness, coma and possible death due to respiratory failure. Substance is a hepatotoxin and is capable of producing a toxic effect on the liver.
Inhalation:
May cause liver and kidney damage. Exposure produces central nervous system depression. May be harmful if inhaled.
Chronic:
Prolonged or repeated skin contact may cause dermatitis. Chronic ingestion may cause effects similar to those of acute ingestion. May cause liver and kidney damage. May cause cancer according to animal studies. Chronic exposure may cause visual disturbances. Carbon tetrachloride is a CNS depressant.
SECTION 4 - FIRST AID MEASURES Eyes:
In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid immediately. Wash clothing before reuse.
Ingestion:
Potential for aspiration if swallowed. Get medical aid immediately. Do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person.
Inhalation:
Poison material. If inhaled, get medical aid immediately. Remove victim to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Material will not burn. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
Extinguishing Media:
Use extinguishing media most appropriate for the surrounding fire.
SECTION 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Isolate area and deny entry. Provide ventilation.
SECTION 7 - HANDLING and STORAGE Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Do not breathe vapor. Use only with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use only under a chemical fume hood. Personal Protective Equipment
Eyes:
Wear chemical goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary.
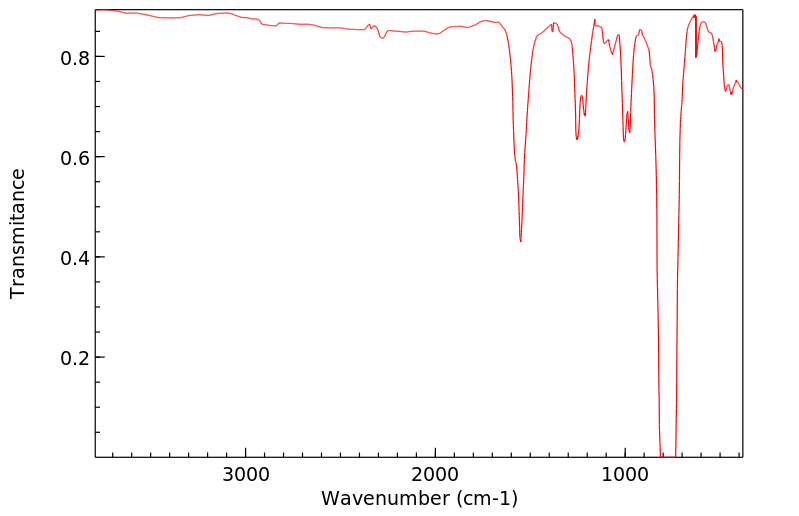
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid
Color: clear, colorless
Odor: chloroform-like
pH: Not available.
Vapor Pressure: 91 mm Hg @ 20 deg C
Viscosity: 0.97 PAS 20 deg C
Boiling Point: 76 deg C @ 760 mm Hg
Freezing/Melting Point: -23 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: >100 deg C
Solubility in water: Insoluble.
Specific Gravity/Density: 1.5900 g/cm3
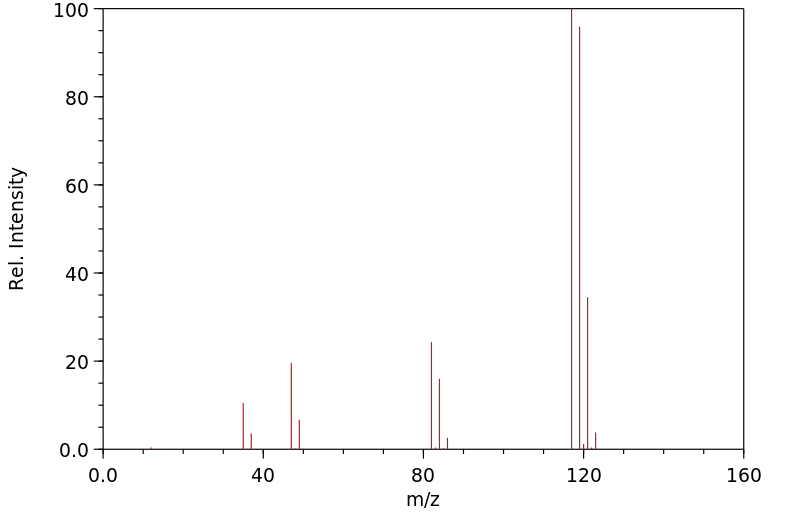
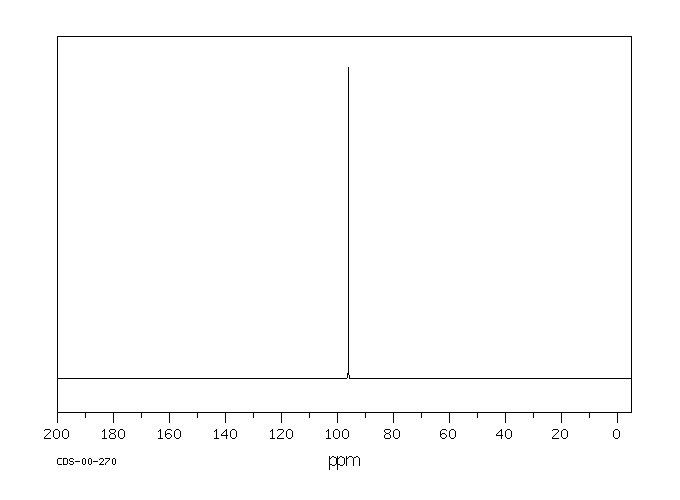
Molecular Formula: CCl4
Molecular Weight: 153.82
SECTION 10 - STABILITY AND REACTIVITY Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Light, excess heat.
Incompatibilities with Other Materials:
Alkali metals, powdered aluminum, powdered magnesium, zinc powder, ethylene, allyl alcohol, barium, fluorine, dimethylformamide, powered beryllium, decaborane, potassium tert-butoxide.
Hazardous Decomposition Products:
Hydrogen chloride, chlorine, phosgene, carbon monoxide, carbon dioxide, chlorine dioxide, which may be spontaneously explosive.
Hazardous Polymerization: Will not occur.
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 56-23-5: FG4900000
LD50/LC50:
CAS# 56-23-5: Dermal, guinea pig: LD50 = >9400 uL/kg; Draize test,
rabbit, eye: 2200 ug/30S Mild; Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 4 mg Mild; Draize test, rabbit,
skin: 500 mg/24H Mild; Inhalation, mouse: LC50 = 9526 ppm/8H;
Inhalation, rat: LC50 = 8000 ppm/4H; Oral, mouse: LD50 = 8263 mg/kg;
Oral, rabbit: LD50 = 5760 mg/kg; Oral, rat: LD50 = 2350 mg/kg; Skin,
rabbit: LD50 = >20 gm/kg; Skin, rat: LD50 = 5070 mg/kg.
Carcinogenicity:
Carbon Tetrachloride - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
Other:
See actual entry in RTECS for complete information.
SECTION 12 - ECOLOGICAL INFORMATION Ecotoxicity:
Fish: Fathead Minnow: LC50 = 20.8-41.4 mg/L; 96 Hr.; Flow-through; 21.7 degrees CFish: Bluegill/Sunfish: LC50 = 27-125 mg/L; 96 Hr.; Static Conditions; 23 degrees CBacteria: Phytobacterium phosphoreum: EC50 = 6.0 mg/L; Not available; Microtox testBacteria: Phytobacterium
phosphoreum: EC50 = 33.0 mg/L; 30 minutes; Microtox test
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA
Shipping Name: CARBON TETRACHLORIDE
Hazard Class: 6.1
UN Number: 1846
Packing Group: II IMO
Shipping Name: CARBON TETRACHLORIDE
Hazard Class: 6.1
UN Number: 1846
Packing Group: II RID/ADR
Shipping Name: CARBON TETRACHLORIDE
Hazard Class: 6.1
UN Number: 1846
Packing group: II
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: T N
Risk Phrases:
R 23/24/25 Toxic by inhalation, in contact with skin and if swallowed. R 40 Limited evidence of a carcinogenic effect. R 59 Dangerous for the ozone layer.
R 48/23 Toxic : danger of serious damage to health by prolonged exposure through inhalation. R 52/53 Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray. S 36/37 Wear suitable protective clothing and gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). S 59 Refer to manufacturer/supplier for information on recovery/recycling. S 61 Avoid release to the environment. Refer to special instructions/Safety data sheets. WGK (Water Danger/Protection) CAS# 56-23-5: 3 United Kingdom Occupational Exposure Limits United Kingdom Maximum Exposure Limits Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 56-23-5 is not listed on Canada's Ingredient Disclosure List. Exposure Limits CAS# 56-23-5: OEL-ARAB Republic of Egypt:TWA 5 ppm (30 mg/m3);Skin OEL-AUSTRALIA:TWA 5 ppm (30 mg/m3);Skin;Carcinoge OEL-BELGIUM:TWA 5 ppm (31 mg/m3);Skin;Carcinogen OEL-CZECHOSLOVAKIA:TWA 10 mg/m3;STEL 20 mg/m3 OEL-DENMARK:TWA 2 ppm (13 mg/m3);Skin OEL-FINLAND:TWA 5 ppm (31 mg/m3);STEL 10 ppm (63 mg/m3);Skin;CAR OEL-FRANCE:TWA 2 ppm (12 mg/m3);STEL 10 ppm (60 mg/m3) OEL-GERMANY:TWA 10 ppm (65 mg/m3);Skin;Carcinogen OEL-HUNGARY:STEL 10 mg/m3;Skin;Carcinogen OEL-INDIA:TWA 5 ppm (30 mg/m3);Skin;Carcinogen OEL-JAPAN:TWA 10 ppm (63 mg/m3);Skin;Carcinogen OEL-THE NETHERLANDS:TWA 2 ppm (12.6 mg/m3);Skin OEL-THE PHILIPPINES:TWA 10 ppm (65 mg/m3);Skin OEL-POLAND:TWA 20 mg/m3 OEL-RUSSIA:TWA 10 ppm;STEL 20 mg/m3 OEL-SWEDEN:TWA 2 ppm (13 mg/m3);STEL 3 ppm (19 mg/m3);Skin;CAR OEL-SWITZERLAND:TWA 5 ppm (30 mg/m3);STEL 10 ppm (60 mg/m3);Skin OEL-THAILAND:TWA 10 ppm;STEL 25 ppm OEL-UNITED KINGDOM:TWA 10 ppm (65 mg/m3);STEL 20 ppm;Skin OEL IN BULGARIA, COLOMBIA, JORDAN, KOREA check ACGIH TLV OEL IN NEW ZEALAND, SINGAPORE, VIETNAM check ACGI TLV US FEDERAL TSCA CAS# 56-23-5 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION MSDS Creation Date: 7/20/1999 Revision #4 Date: 11/06/2002 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION N/A