3-氯丁酸甲酯 | 817-76-5
中文名称
3-氯丁酸甲酯
中文别名
克罗米通杂质11
英文名称
2-chloropropyl methyl ester
英文别名
3-chloro-butyric acid methyl ester;3-Chlor-buttersaeure-methylester;3-chloro-butanoic acid methyl ester;Methyl 3-chlorobutanoate
CAS
817-76-5
化学式
C5H9ClO2
mdl
MFCD00018873
分子量
136.578
InChiKey
HDQHWBXUKZBPDN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:48-51 °C(Press: 13 Torr)
-
密度:1.077 g/cm3
-
保留指数:870;873;881;859;864;869
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : METHYL 3-CHLOROBUTYRATE
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed. Irritating to eyes, respiratory system and skin. Harmful to aquatic organisms, may
cause long-term adverse effects in the aquatic environment.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
R36/37/38 Irritating to eyes, respiratory system and skin.
R52/53 Harmful to aquatic organisms, may cause long-term adverse effects in
the aquatic environment.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S36/37 Wear suitable protective clothing and gloves.
S61 Avoid release to the environment. Refer to special instructions/ Safety
data sheets.
Caution - substance not yet tested completely.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C5H9ClO2
Molecular Weight : 136,58 g/mol
Component Concentration
METHYL 3-CHLOROBUTYRATE
-
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Evacuate personnel to safe areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 155 - 156 °C at 1.010 hPa
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 1,306
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Specific target organ toxicity - single exposure
no data available
no data available
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
Harmful to aquatic life.
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
上下游信息
反应信息
-
作为反应物:描述:3-氯丁酸甲酯 在 sodium sulfide 、 alkaline aqueous solution 作用下, 生成 2,6-dimethyl-4-oxo-tetrahydro-thiopyran-3-carboxylic acid methyl ester参考文献:名称:Arndt; Bekir, Chemische Berichte, 1930, vol. 63, p. 2393,2396摘要:DOI:
-
作为产物:参考文献:名称:Henry, Chemisches Zentralblatt, 1898, vol. 69, # II, p. 273摘要:DOI:
文献信息
-
Method for producing organic compounds by substituting halogen atoms申请人:MITSUI CHEMICALS, INC.公开号:EP1486479A1公开(公告)日:2004-12-15The invention pertains to a method in which a halogen atom of an organic compound is replaced with a group derived from a nucleophilic agent, at high yield and high efficiency, by the following method which includes a step of reacting the nucleophilic agent with an organic material having a halogen atom bonded to a carbon atom having four σ bonds, more specifically: a method for producing an organic compound having Q, the method including a step of reacting a compound represented by general formula (2) with an organic starting material having at least one halogen atom bonded to a carbon atom having four σ bonds so as to replace the halogen atom in the organic starting material with Q: MQa (2) (wherein M represents an alkali metal atom, an alkali earth metal atom, or a rare earth metal atom; Q represents a moiety of an inorganic acid or an active hydrogen compound derived by eliminating a proton, wherein Q is a halogen atom different from the halogen atom in the organic starting material having the halogen atom bonded to the carbon atom having the four σ bonds; and a represents an integer of 1 to 3) in the presence of a compound represented by general formula (1) (wherein Z- represents an anion derived by eliminating a proton from an inorganic acid or an active hydrogen compound; R2 is the same or different; R2 each independently represent a C1-C10 hydrocarbon group or two R2 on the same nitrogen atom may be bonded with each other to form a ring with the nitrogen atom).这项发明涉及一种方法,其中有机化合物中的卤素原子被来自亲核试剂的基团取代,且产率高效率高,通过以下方法实现,包括以下步骤:将亲核试剂与具有与碳原子形成四个σ键的卤素原子相结合的有机材料反应的步骤,更具体地说:一种用于生产具有Q的有机化合物的方法,包括以下步骤:将由通式(2)表示的化合物与至少一个卤素原子与碳原子形成四个σ键的有机起始材料反应,以将有机起始材料中的卤素原子替换为Q: MQa (2) (其中M代表碱金属原子、碱土金属原子或稀土金属原子;Q代表由消除质子衍生的无机酸或活性氢化合物的基团,其中Q是不同于有机起始材料中卤素原子的卤素原子,该卤素原子与具有四个σ键的碳原子相结合;a表示1到3的整数),在通式(1)表示的化合物的存在下 (其中Z-代表由无机酸或活性氢化合物中消除质子衍生的阴离子;R2相同或不同;R2各自独立地表示C1-C10烃基,或者两个R2在同一氮原子上可能与彼此结合形成与氮原子的环)。
-
γ-Substituted Secondary Organoalkaline Compounds and their Chlorinated Precursors: Synthetic Applications作者:José Barluenga、Josefa Flórez、Miguel YusDOI:10.1055/s-1985-31362日期:——The prepartion of γ-functionalised secondary organolkaline metal compounds starting from methyl 3-chlorobutanoate (obtained by addition of hydrogen chloride to commercially available methyl trans-2-butenoate) is described. Reactions of these metallated compounds with suitable electrophilic reagents leads to a variety of tertiary alcohol derivatives.
-
13C NMR, 1H NMR and IR spectra of a series of monochloroesters of aliphatic short chain carboxylic acids作者:Maija T. Pitkänen、Ilpo O.O. Korhonen、Jorma N.J. KorvolaDOI:10.1016/s0040-4020(01)92425-1日期:1981.1chemical shifts for 14 isomeric monochloroesters of aliphatic carboxylic acids from propanoic acid to hexanoic acid have been determined. Comparisons are made with the literature values for methyl monochloro-octanoate isomers. Substituent effects for all positions are given. Characteristic IR absorption bands are presented and comparisons are made with regard to the isomeric structure. Connections
-
Röntgenkontrastmittel auf der Basis jodierter Phenoxyfettsäuren作者:H. CassebaumDOI:10.1002/ardp.19592921102日期:——Die Darstellung mono‐, di‐, tri‐ und tetrajodierter Phenoxyfettsäuren durch Kondensation jodierter Phenole mit geeigneten Halogenfettsäureestern oder durch Jodieren von Phenoxyfettsäuren mit JCL wird beschrieben. Bessere Herstellungsmethoden bekannter Jodphenole und die Synthesen einiger di‐, tri‐ und tetrajodierter Phenole werden angegeben. Zwei Versuche zur Gewinnung der Tetrajod‐m‐aminobenzoesäureDie Darstellung mono-, di-, tri- und tetrajodierter Phenoxyfettsäuren durch Kondensation jodierter Phenole mit geeigneten Halogenfettsäureestern oder durch Jodieren von Phenoxyfettsäuren mit JCL wird beschrieben。Bessere Herstellungsmethoden bekannter Jodphenole und die Synthesen einiger di-, tri- und tetrajodierter Phenole werden angegeben。Zwei Versuche zur Gewinnung der Tetrajod-m-aminobenzoesäure
-
A straightforward synthesis of both enantiomers of allo-norcoronamic acids and allo-coronamic acids, by asymmetric Strecker reaction from alkylcyclopropanone acetals作者:Antoine Fadel、Aballache KhesraniDOI:10.1016/s0957-4166(97)00633-2日期:1998.1Methylcyclopropanone hemiacetal (2S)-3a underwent the asymmetric Strecker reaction induced by a chiral amine to provide a useful synthesis of enantiomerically pure (1R,2S)-(+)-allo-norcoronamic acid 1 in good yield and high enantiomeric excess. From racemic alkyl hemiacetal (±)-3, the same methodology also constituted a useful way to prepare both (+)-1 and (−)-1 and (+)-allo-coronamic acid 2 and its
表征谱图
-
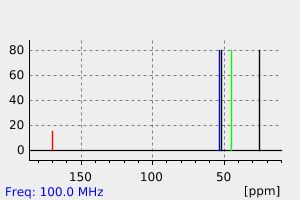
氢谱1HNMR
-
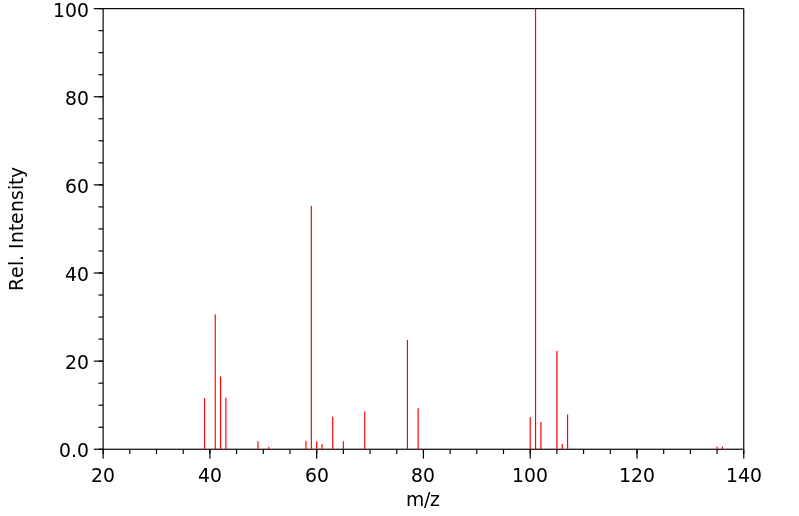
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯