2,3,5,6-四氟苯肼 | 653-11-2
中文名称
2,3,5,6-四氟苯肼
中文别名
2,3,5,6-四氟苯基肼
英文名称
2,3,5,6-tetrafluorophenylhydrazine
英文别名
2,3,5,6-Tetrafluor-phenylhydrazin;(2,3,5,6-tetrafluorophenyl)hydrazine
CAS
653-11-2
化学式
C6H4F4N2
mdl
MFCD00007575
分子量
180.105
InChiKey
TYMFVEOXNZYYTF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:91-93 °C(lit.)
-
沸点:136.3±40.0 °C(Predicted)
-
密度:1.4197 (estimate)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:38
-
氢给体数:2
-
氢受体数:6
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S23,S26,S37/39
-
危险类别码:R20/21
-
WGK Germany:3
-
海关编码:2928000090
-
危险品运输编号:UN 2811
-
储存条件:常温常压下存储。
SDS
| Name: | 2 3 5 6-Tetrafluorophenylhydrazine 97+% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 653-11-2 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 653-11-2 | 2,3,5,6-Tetrafluorophenylhydrazine | 97+% | 211-494-4 |
Risk Phrases: 20/21
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 653-11-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Flakes
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 91.00 - 93.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H4F4N2
Molecular Weight: 180.10
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas, nitrogen.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 653-11-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3,5,6-Tetrafluorophenylhydrazine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21 Harmful by inhalation and in contact with
skin.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
WGK (Water Danger/Protection)
CAS# 653-11-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 653-11-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 653-11-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (2,3,5,6-四氟-4-肼基苯基)肼 1,4-dihydrazino-tetrafluorobenzene 2248-05-7 C6H6F4N4 210.134
反应信息
-
作为反应物:描述:2,3,5,6-四氟苯肼 在 phosphorus pentoxide 作用下, 以 乙醇 、 氯仿 为溶剂, 反应 32.0h, 生成 4-(3-Hydroxypropyl)-1-(2,3,5,6-tetrafluorophenyl)-5-trifluoromethylpyrazole参考文献:名称:Synthesis of fluorinated pyrazole derivatives from β-alkoxyvinyl trifluoroketones摘要:1.1.1-Trifluoro-4-ethoxy-3-butane-2-one, 3-trifluoroacetyl-3, 4-dihydro-2H-pyran or furan reacted readily with pentafluorophenylhydrazine or per(poly)fluoroacectylhydrazine RtCO-NHNH2 (R-1: BrCF2, C3F7) to give N-substituted-5-hydroxy-5-trifluoromrthyl I heterocycles Y-N-N=CH-CH(R)C(OH)CF3 (Y: H, Ar-f - or RtCO), which were dehydrated by treatment with P2O5 or SOCl2 to form N-substituted 5-trifluoromethyl pyrazoles Y-N-N=CH-C(R)=C CF3 (Y: H, Ar-f - or RtCO) in good yields.[GRAPHICS](C) 2001 Elsevier Science B.V. All rights reserved.DOI:10.1016/s0022-1139(00)00348-1
-
作为产物:参考文献:名称:多氟苯基取代的 Blatter 自由基:合成和结构-性能相关性摘要:布拉特自由基是极其稳定的有机缺电子顺磁体,具有作为多种应用中的自旋轴承构件的良好潜力。这些基团的堆积模式和分子构象适合于获得独特的磁和电子特性。在此,在系统探索氟化布拉特自由基固有的“结构-性质”相关性的框架内,1-(2,3,4-三氟苯基)-(2a)和1-(2,3,5,6-四氟苯基) )-3-苯基-1,4-二氢苯并[e][1,2,4]三嗪-4-基 (2b) 被合成并完全表征。发现基团2a和2b的晶体结构由两个不同的中心对称二聚体组成,苯并三嗪基部分的原子之间的分子间距离相对较短。 2-300 K 范围内的 SQUID 磁力测量表明,这两个自由基的晶体,就像之前合成的 1-(五氟苯基)-3-苯基-1,4-二氢苯并[e][1,2,4]三嗪的晶体一样-4-基(2c),以相当强的反铁磁相互作用为主。对于这两种二聚体类型,自旋非限制对称性破缺 DFT 计算预测相似的参数 J 相差不到 20%。随后使用理论预测的磁性基序拟合DOI:10.1021/acs.cgd.4c00537
-
作为试剂:描述:参考文献:名称:一种催化氧化芳硼类化合物制备酚的方法摘要:本发明公开了一种催化氧化芳硼类化合物合成酚类化合物的方法,在溶剂醇或醇的水溶液中,在碱的作用下,加入水合肼或肼类化合物催化剂,催化氧化芳硼类化合物直接制备酚类化合物。本发明的制备酚类化合物的方法,催化剂是廉价的水合肼或肼类化合物,氧化剂为常压下的空气或氧气,反应无需金属催化剂且活性好,底物来源广泛且稳定,底物敏感官能团相容性好且适用范围广。在优化的反应条件之下,目标产品分离收率高达99%。公开号:CN103936538B
文献信息
-
N-arylheteroaromatic products compositions containing them and use thereof申请人:Le-Brun Alain公开号:US20050130989A1公开(公告)日:2005-06-16N-Arylheteroaromatic products, compositions containing them and use thereof. The present invention relates to novel chemical compounds, particularly to novel N-arylheteroaromatic products, to compositions containing them and to their use as medicinal products, in particular in oncology.N-芳基杂芳产物,含有它们的组合物及其用途。本发明涉及新型化合物,特别是新型N-芳基杂芳产物,含有它们的组合物以及它们作为药用产品的用途,特别是在肿瘤学中的用途。
-
On the application of the extended Fujita–Nishioka equation to polysubstituted systems. A kinetic study of the rearrangement of several poly-substituted Z-arylhydrazones of 3-benzoyl-5-phenyl-1,2,4-oxadiazole into 2-aryl-4-benzoylamino-5-phenyl-1,2,3-triazoles in dioxane/water作者:Francesca D'Anna、Fiammetta Ferroni、Vincenzo Frenna、Susanna Guernelli、Camilla Zaira Lanza、Gabriella Macaluso、Vitalba Pace、Giovanni Petrillo、Domenico Spinelli、Raffaella SpisaniDOI:10.1016/j.tet.2004.10.054日期:2005.1or penta-substituted Z-arylhydrazones of 3-benzoyl-5-phenyl-1,2,4-oxadiazole (1a–18a) into the relevant 2-aryl-4-benzoylamino-5-phenyl-1,2,3-triazoles (1b–18b) have been determined in 1:1 (v:v) dioxane/water in a wide range of pS+ (3.80–12.50) at different temperatures. The kinetic data obtained have been correlated with those previously collected for the rearrangement of ortho-, meta- and para-substituted3-苯甲酰基-5-苯基-1,2,4-恶二唑(1a – 18a)的几个二,三,四或五取代的Z-芳基hydr重排成相关的2-芳基-4-的重排率苯甲酰氨基-5-苯基-1,2,3-三唑(1b – 18b)已在不同温度下在1:1 p(v:v)二恶烷/水中以较大的p S +(3.80–12.50)测定。获得的动力学数据已与先前收集的用于重排邻,间和对位取代的Z-芳基azo(19a – 38a)的数据相关。)通过藤田和西冈提出,从而在考虑空间位线性自由能关系(LFER)的延伸的装置(Ë小号)和场(˚F ø在除了正常的电子效应)邻近效应(σ邻,间, p)。良好的相关系数被计算出(- [R ≥0.999),具有易感性常数(ρ,δ和˚F)接近先前对所获得的那些19A - 38A,当仅不包括数据的2,6-双-邻-取代的ž -arylhydrazones 11a和16 –图18a显示了比可预见的高得多的反应性,证
-
Antibacterial dimeric copper(II) complexes with chromone-derived compounds作者:Zahid H. Chohan、Mohammad S. Iqbal、Syed K. Aftab、A. RaufDOI:10.3109/14756366.2011.585135日期:2012.4.1A new series of six chromone-derived compounds and their Cu(II) complexes have been synthesized and characterized by their physical, spectral and analytical data. The ligands and their Cu(II) complexes were screened for their in vitro antibacterial activity against four Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri) and two Gram-positive (Bacillus subtilis
-
Salicylic Acid Based Small Molecule Inhibitor for the Oncogenic Src Homology-2 Domain Containing Protein Tyrosine Phosphatase-2 (SHP2)作者:Xian Zhang、Yantao He、Sijiu Liu、Zhihong Yu、Zhong-Xing Jiang、Zhenyun Yang、Yuanshu Dong、Sarah C. Nabinger、Li Wu、Andrea M. Gunawan、Lina Wang、Rebecca J. Chan、Zhong-Yin ZhangDOI:10.1021/jm901645u日期:2010.3.25homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) plays a pivotal role in growth factor and cytokine signaling. Gain-of-function SHP2 mutations are associated with Noonan syndrome, various kinds of leukemias, and solid tumors. Thus, there is considerable interest in SHP2 as a potential target for anticancer and antileukemia therapy. We report a salicylic acid based combinatorial library approachSrc homology-2 域包含蛋白酪氨酸磷酸酶-2 (SHP2) 在生长因子和细胞因子信号传导中起关键作用。功能获得性 SHP2 突变与 Noonan 综合征、各种白血病和实体瘤有关。因此,人们对 SHP2 作为抗癌和抗白血病治疗的潜在靶点有相当大的兴趣。我们报告了一种基于水杨酸的组合文库方法,旨在结合活性位点和附近独特的亚袋,以提高亲和力和选择性。文库的筛选导致鉴定出具有高效细胞活性的 SHP2 抑制剂 II-B08(化合物9)。化合物9阻断生长因子刺激的 ERK1/2 激活和造血祖细胞增殖,提供支持证据表明 SHP2 的化学抑制可能对抗癌和抗白血病治疗有治疗作用。与9复合的 SHP2 结构的 X 射线晶体学分析揭示了可用于获得更有效和选择性 SHP2 抑制剂的分子决定因素。
-
Synthesis of polyfluorinated arylhydrazines, arylhydrazones and 3-methyl-1-aryl-1H-indazoles作者:Larisa Politanskaya、Irina Bagryanskaya、Evgeny TretyakovDOI:10.1016/j.jfluchem.2018.06.011日期:2018.10derivatives, while E-isomers were not active and were isolated unchanged. Molecular and crystal structures of prepared polyfluorinated (E)-arylhydrazones as well as selected 3-methyl-1-aryl-1H-indazoles were solved by the X-ray diffraction analysis. Meanwhile, the polyfluorinated acetophenone reacted with hydrazine in THF in the absence of a catalyst through a condensation–nucleophilic substitution
表征谱图
-
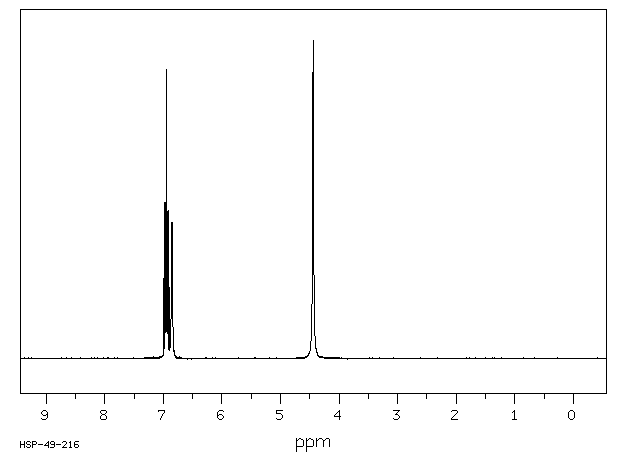
氢谱1HNMR
-
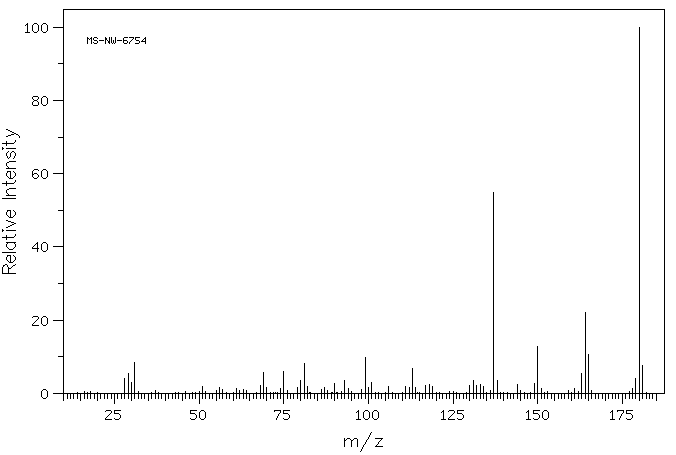
质谱MS
-
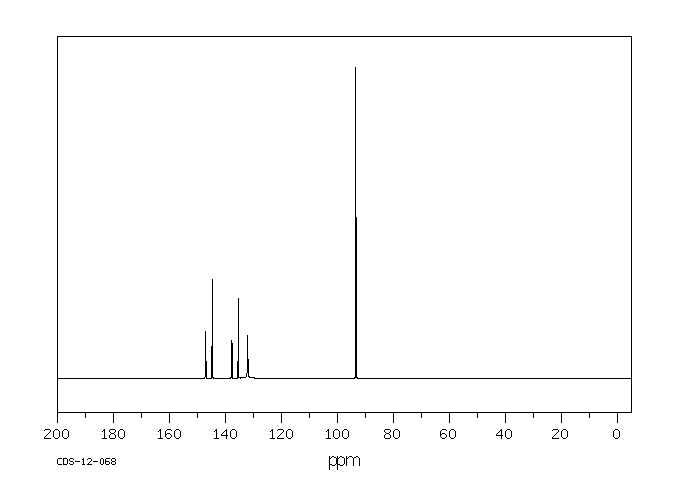
碳谱13CNMR
-
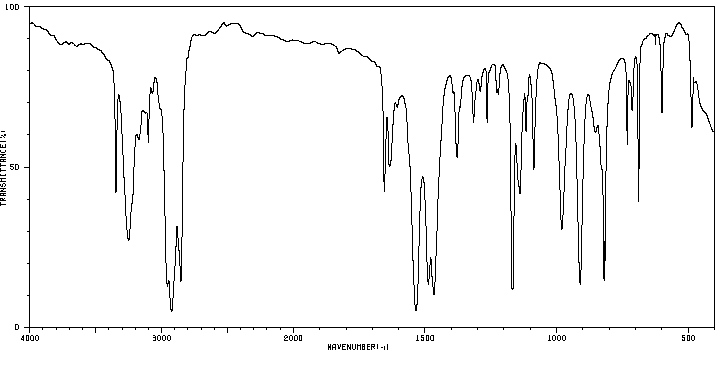
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫