异牡荆黄素 | 38953-85-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:257-258 °C
-
沸点:807.0±65.0 °C(Predicted)
-
密度:1.686±0.06 g/cm3(Predicted)
-
溶解度:DMF:30mg/mL; DMSO:30mg/mL; DMSO:PBS (pH 7.2) (1:5):0.16 mg/mL
-
LogP:1.280 (est)
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:31
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:177
-
氢给体数:7
-
氢受体数:10
安全信息
-
WGK Germany:3
-
海关编码:29329990
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:-20°C
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Isovitexin
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 38953-85-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : Saponaretin
Formula : C21H20O10
Molecular Weight : 432,38 g/mol
CAS-No. : 38953-85-4
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapours, mist or gas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an industrial hygienist and safety officer familiar with the specific situation of
anticipated use by our customers. It should not be construed as offering an approval for any
specific use scenario.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: powder
Colour: light yellow
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing 220 - 221 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
异牡荆黄素是从亚洲水稻中得到的一种黄酮类物质,具有抗氧化和抗炎的活性。其作用类似于 JNK1/2 抑制剂,能够抑制 NF-κB 的活化。
生物活性异牡荆黄素可保护细胞免受脂多糖(LPS)诱导的氧化损伤,通过抑制细胞内 ROS 生成;同时还能减弱过氧化氢(H₂O₂)对细胞存活的影响。在 RAW 264.7 细胞中,200 μg/mL 的异牡荆黄素显示显著的细胞毒性,而 Isovitexin (0-100 μg/mL) 结合 LPS (2 μg/mL) 并不表现出细胞毒性。异牡荆黄素(25, 50 μg/mL)能抑制由脂多糖诱导的 TNF-α、IL-6、iNOS 和 COX-2 的水平上升,还能抑制 RAW 264.7 细胞中 IκBα 的磷酸化和降解,其效果与 JNK1/2 抑制剂一致。
靶点| JNK1 | |
| JNK2 | |
| NF-κB |
在体外,异牡荆黄素 (Isovitexin) 对 RAW 264.7 细胞的保护作用表现在 LPS 刺激下抑制 ROS 的生成,并减弱 H₂O₂ 对细胞存活的影响。200 μg/mL 的 Isovitexin 显示显著细胞毒性,而 Isovitexin (0-100 μg/mL) 结合 2 μg/mL 的 LPS 并不表现出细胞毒性。异牡荆黄素(25, 50 μg/mL)能抑制脂多糖诱导的 TNF-α、IL-6、iNOS 和 COX-2 的水平上升,还能抑制 RAW 264.7 细胞中 IκBα 的磷酸化和降解。
体内研究在体内实验中,异牡荆黄素 (50 和 100 mg/kg, i.p.) 可减轻 LPS 刺激小鼠肺组织的病理变化,并降低炎症细胞计数。异牡荆黄素 (50 和 100 mg/kg, i.p.) 能通过减少 TNF-α 和 IL-6 的产生、ROS 的生成、MPO 和 MDA 内容,增加 SOD 和 GSH 的水平来保护 LPS 刺激的小鼠免受炎症和氧化应激的影响,并有效抑制 LPS/D-gal 引起的 iNOS 和 COX-2 的蛋白表达。异牡荆黄素(25, 50, 100 mg/kg)在 LPS/D-gal 引发的小鼠肝损伤中表现出剂量依赖性的生存率降低作用,同时还能抑制 NF-κB 活化并上调由 LPS/D-gal 导致的 Nrf2 和 HO-1 表达。
化学性质淡黄色结晶粉末,可溶于甲醇、乙醇、DMSO 等有机溶剂。来源于山楂叶、竹叶、新西兰牡荆 Vitex lucens、滨牡荆 Virex littoRAlis、皂荚 Gleditsia sinensis [Syn.Gleditsia horrida]、酸角 Tamarindus indica、亚麻 Linum usitatissimum、獐牙菜 Swertia PSeudochinensis。
用途异牡荆黄素具有抗炎、抗氧化、抗肿瘤和降血压的作用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异牡荆素-2''-O-葡萄糖苷 isovitexin-2''-O-β-D-glucopyranoside 60767-80-8 C27H30O15 594.526 —— isovitexin 2"-O-sulfate 1173925-23-9 C21H20O13S 512.448 —— 7-benzyloxy-2-(4-benzyloxyphenyl)-5-hydroxy-6-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)-4H-1-benzopyran-4-one —— C63H56O10 973.132 —— 7-O-trans-sinapoylisovitexin —— C32H30O14 638.582 牡荆素 vitexin 3681-93-4 C21H20O10 432.384 —— 7-O-trans-sinapoylisovitexin 4'-O-β-D-glucopyranoside —— C38H40O19 800.724 —— 6''-(2-O-trans-sinapoyl-β-D-glucopyranosyl)-7-O-trans-sinapoylisovitexin —— C49H50O23 1006.92 —— 6''-(2-O-trans-sinapoyl-β-D-glucopyranosyl)-7-O-trans-sinapoylisovitexin 4'-O-β-D-glucopyranoside —— C55H60O28 1169.06 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-[(4aR,6S,7R,8R,8aS)-7,8-dihydroxy-2-phenyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one 1233717-59-3 C28H24O10 520.493 —— isovitexin 2"-O-sulfate 1173925-23-9 C21H20O13S 512.448 —— chafuroside A —— C21H18O9 414.369 —— [(4aR,6S,7R,8S,8aS)-6-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-6-yl]-8-hydroxy-2-phenyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-7-yl] hydrogen sulfate 1233717-60-6 C28H24O13S 600.557 牡荆素 vitexin 3681-93-4 C21H20O10 432.384 —— vitexin 3681-93-4 C21H20O10 432.384 —— chafuroside B 866737-00-0 C21H18O9 414.369
反应信息
-
作为反应物:参考文献:名称:EP1731522摘要:公开号:
-
作为产物:描述:5,7,4'-trihydroxy-6-C-(β-D-glucopyranosyl)flavone peracetate 在 sodium methylate 作用下, 以 甲醇 为溶剂, 反应 5.0h, 以87%的产率得到异牡荆黄素参考文献:名称:Regioselective synthesis of di-C-glycosylflavones possessing anti-inflammation activities摘要:采用三种方法合成了多种具有相同或不同糖基的6,8-二-C-糖苷黄酮。发现一些C-糖苷化合物的抗炎活性优于其母体黄酮。其中,6,8-二-C-葡萄糖苷阿比金(称为维塞宁-2)对TNF-α表达和NO产生的抑制作用分别具有6.8和5.2 μM的IC50值。DOI:10.1039/c0ob00011f
文献信息
-
[EN] COMPOUNDS EFFECTIVE IN TREATING HEPATOTOXICITY AND FATTY LIVER DISEASES AND USES THEREOF<br/>[FR] COMPOSÉS EFFICACES POUR TRAITER L'HÉPATOTOXICITÉ ET DES STÉATOSES HÉPATIQUES, ET UTILISATIONS DE CEUX-CI申请人:SINEW PHARMA INC公开号:WO2017050298A1公开(公告)日:2017-03-30The present invention relates to compounds effective in treating hepatotoxicity and fatty liver diseases and uses thereof.本发明涉及用于治疗肝毒性和脂肪肝疾病的化合物及其用途。
-
The extractives of Vitex lucens—I作者:Lindsay H. Briggs、R.C. CambieDOI:10.1016/0040-4020(58)80022-8日期:1958.1Vitexin, isolated from Vitex lucens, has been formulated as C21H20O10 and, in agreement with recent workers, structure (I) has been assigned to the compound. β-sitosterol has been isolated from the heartwood.分离自Vitex lucens的Vitexin配制成C 21 H 20 O 10,并与最近的工作人员一致,将结构式(I)分配给该化合物。β-谷甾醇已从心材中分离出来。
-
C-Glycosyl Flavones from Two Eastern Siberian Species of Silene作者:D. N. Olennikov、N. K. ChirikovaDOI:10.1007/s10600-019-02768-7日期:2019.7Flavonoids from Silene aprica Turcz. and S. samojedorum (Sambuk) Oxelman (Caryophyllaceae) growing in Baikal region were studied for the first time. A total of 14 compounds including three new compounds 1–3 were isolated. Their structures were established using UV, IR, and NMR spectroscopy and mass spectrometry. Apigenin-6-C-(2″-O-α-L-arabinopyranosyl-6″-O-acetyl)-β-D-glucopyranoside (sileneside A, 1) and apigenin-6-C-(2″-O-β-D-glucopyranosyl-6″-O-acetyl)-β-D-glucopyranoside (sileneside B, 2) were observed in S. aprica; 1 and luteolin-6-C-(2″-O-α-L-arabinopyranosyl-6″-O-acetyl)-β-D-glucopyranoside (sileneside C, 3), in S. samojedorum.来自 Silene aprica Turcz 的黄酮类化合物。首次研究了贝加尔湖地区生长的 S. samojedorum (Sambuk) Oxelman (石竹科)。共分离出 14 种化合物,其中包括 3 种新化合物 1-3。它们的结构是通过紫外、红外、核磁共振光谱和质谱确定的。芹菜素-6-C-(2″-O-α-L-阿拉伯吡喃糖基-6″-O-乙酰基)-β-D-吡喃葡萄糖苷(硅烯苷A,1)和芹菜素-6-C-(2″-O-在 S. aprica 中观察到 β-D-吡喃葡萄糖基-6″-O-乙酰基)-β-D-吡喃葡萄糖苷 (sileneside B, 2); 1 和木犀草素-6-C-(2″-O-α-L-阿拉伯吡喃糖基-6″-O-乙酰基)-β-D-吡喃葡萄糖苷(硅烯苷 C,3),在 S. samojedorum 中。
-
Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases申请人:Scaramuzzino, Giovanni公开号:EP1336602A1公开(公告)日:2003-08-20New pharmaceutical compounds of general formula (I): F-(X)q where q is an integer from 1 to 5, preferably 1; -F is chosen among drugs described in the text, -X is chosen among 4 groups -M, -T, -V and -Y as described in the text. The compounds of general formula (I) are nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without hypotensive side effects and for this reason they are useful for the preparation of medicines for prevention and treatment of inflammatory, ischemic, degenerative and proliferative diseases of musculoskeletal, tegumental, respiratory, gastrointestinal, genito-urinary and central nervous systems.
-
Flavonoid pattern inheritance in the allopolyploid Spartina anglica – Comparison with the parental species S. maritima and S. alterniflora作者:Micheline Grignon-Dubois、Xavier De Montaudouin、Bernadette RezzonicoDOI:10.1016/j.phytochem.2020.112312日期:2020.6comparison between the three species and determination of the phenolic pattern inheritance in S. anglica. A total of 20 phenolic compounds were detected in the aerial tissues of S. anglica and S. alterniflora, but only seven in S. maritima. They were isolated from their respective crude extracts, and their structures were determined according to spectroscopic data analysis and chemical evidence. They all belong入侵物种 Spartina anglica 起源于欧洲,由非洲-欧洲物种 S. maritima(本地、父系祖先)和引入的北美互花米草(入侵、母系祖先)杂交而成。从植物组织中制备含水甲醇提取物,用于三个物种之间的化学分类比较,并确定 S. anglica 中的酚类遗传。在 S. anglica 和 S. alterniflora 的地上组织中共检测到 20 种酚类化合物,但在 S. maritima 中仅检测到 7 种酚类化合物。它们从各自的粗提物中分离出来,并根据光谱数据分析和化学证据确定了它们的结构。它们都属于黄酮类,其中 13 种被鉴定为 C-糖类黄酮,7 种被鉴定为 O-糖类黄酮。所有这些产品均为首次从S. anglica 中检测到,其中14 种为首次从S. alterniflora 中检测到,其中3 种为首次从S. maritima 中检测到。通过定量 HPLC 测定三种物质中的
表征谱图
-
氢谱1HNMR
-
质谱MS
-
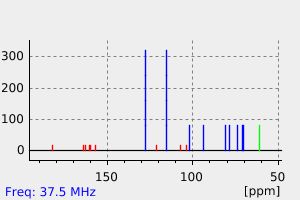
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息