(2,2-二氯-1-甲基环丙基)苯 | 3591-42-2
中文名称
(2,2-二氯-1-甲基环丙基)苯
中文别名
(2,2-二氯-1-甲基环丙基Z)苯;2,2-二氯-1-甲基环丙基苯
英文名称
1,1-dichloro-2-methyl-2-phenylcyclopropane
英文别名
(2,2-dichloro-1-methylcyclopropyl)benzene;2,2-dichloro-1-methyl-1-phenyl-cyclopropane
CAS
3591-42-2
化学式
C10H10Cl2
mdl
MFCD00012260
分子量
201.095
InChiKey
NXYPRVQXSWVEOB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70-72°C 0,8mm
-
密度:1.1720
-
闪点:70-72°C/0.8mm
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
海关编码:2903999090
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | (2 2-Dichloro-1-methylcyclopropyl)benzene 95% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 3591-42-2 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3591-42-2 | (2,2-Dichloro-1-methylcyclopropyl)benz | 95 | 222-734-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Avoid runoff into storm sewers and ditches which lead to waterways.
Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3591-42-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear brown
Odor: benzene-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 75-77C @ 1mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 94 deg C ( 201.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.172
Molecular Formula: C10H10Cl2
Molecular Weight: 201.09
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3591-42-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
(2,2-Dichloro-1-methylcyclopropyl)benzene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3591-42-2: No information available.
Canada
CAS# 3591-42-2 is listed on Canada's DSL List.
CAS# 3591-42-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3591-42-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Cyclic peroxides. 60. Prostanoid endoperoxide model compounds: preparation of 1,2-dioxolanes from cyclopropanes摘要:DOI:10.1021/jo00400a028
-
作为产物:描述:2-苯基-2-丙醇 在 金刚烷 、 potassium tert-butylate 、 potassium hydride 作用下, 以 乙醚 、 二氯甲烷 为溶剂, 反应 180.0h, 生成 (2,2-二氯-1-甲基环丙基)苯参考文献:名称:KOtBu 作为单电子供体?用 CBr4 和 CCl4 重新审视烷烃的卤化摘要:对 KOtBu 和其他叔醇盐可能表现为单电子供体的反应的探索,使我们在金刚烷的存在下探索了它们与四卤甲烷 CX4 的反应。我们最近报道了在这些条件下金刚烷的卤化。这些反应似乎反映了 NaOH 与 CBr4 在相转移条件下的类似已知反应,其中引发的特征是从氢氧根离子到 CBr4 的单电子转移。我们现在报告来自实验和计算研究的证据,即 KOtBu 和其他醇盐试剂不会通过类似的电子转移。相反,醇盐在与 CBr4 或 CCl4 反应时形成次卤酸盐,并且适当次卤酸盐的均裂引发金刚烷的卤化。DOI:10.3390/molecules23051055
文献信息
-
gem-Dihalocyclopropane formation by iron/copper activation of tetrahalomethanes in the presence of nucleophilic olefins. Evidence for a carbene pathway作者:Eric Léonel、Michael Lejaye、Sylvain Oudeyer、Jean Paul Paugam、Jean-Yves NédélecDOI:10.1016/j.tetlet.2004.01.124日期:2004.3The activation of CBr4 and CCl4 by a bimetallic iron/copper couple in acetonitrile is a new, inexpensive, nontoxic and efficient procedure for gem-dibromo- and gem-dichloromethylenation of nucleophilic alkenes. This new route to gem-dihalocyclopropanes involves dihalocarbene species.
-
Kinetics of cyclopropyl radical reactions. 2. Studies on the inversion of cyclopropyl and 1-methylcyclopropyl radicals and on the kinetics of some addition and abstraction reactions of 1-methylcyclopropyl and 1-methoxycyclopropyl radicals作者:Linda J. Johnston、K. U. IngoldDOI:10.1021/ja00269a034日期:1986.4To investigate this possibility we decided to use the same stereospecific pattern of deuterium labeling of the cyclopropyl radical that had been previously employed by Kobayashi and LambertI5 in their (unsuccessful) attempt to trap cyclopropyl before it could invert. These workers generated their labeled radicals from the corresponding labeled cyclopropane carboxylic acid in the Hunsdiecker reaction由于氢隧道效应,反转速度更快。与其他两个基团相比,1-甲氧基环丙基对苯乙烯和 1,4- 环己二烯的反应性较低。无法获得该自由基的 EPR 谱。在第 1 部分)中,我们报道了环丙基自由基在环境温度下在溶液中的一些加成和脱去反应的第一个绝对速率常数。这些通过激光闪光光解获得的动力学数据表明,环丙基的反应性比苯基低,但比伯烷基自由基的反应性强。在某些吸氢反应的后续工作中,我们表明 4 在环境温度下,自由基反应性下降系列:Ph' > Me2C=CH' > c-C3HS > CH3' > RCH2CH2'。我们的结果在很大程度上证实了 Walborsky 的结论 〜基于相对反应性的研究,“环丙基自由基表现为高反应性的快速反转 0 自由基”。已经进行了大量实验,试图确定各种取代的环丙基自由基能够保持其原始构型的程度。 6 这些研究中的大多数都涉及化学捕集,其中由适当取代的环丙基自由基形成的产物被用于确
-
Dihalomethanes as C-H acids in the catalytic two-phase (CTP) system - a new method for the synthesis of gem-dichlorocyclopropanes
-
Selective mono- or dimetalation of arenes by means of superbasic reagents作者:Manfred Schlosser、Hoon Choi Jung、Sadahito TakagishiDOI:10.1016/s0040-4020(01)87763-2日期:1990.1of two mono-and ten di-substituted derivatives. - Alkyl groups, as present in tert-butylbenzene, retard the metalation at both m- and p- positions, while trialkylsilyl groups deactive only m-positions. In either case exclusive monosubstitution occurs. -Perdeuterobenzene undergoes metalation and subsequent electrophilic mono-or disubstitution to afford isotope labeled compounds with moderate, through
-
Synthesis of 1,1-Bis(trimethylstannyl)cyclopropanes by the S<sub>RN</sub>1 Mechanism作者:Javier F. Guastavino、Roberto A. RossiDOI:10.1021/om801104e日期:2009.4.27dichlorocarbene to alkenes, with Me3Sn− anions are reported. The process is described in terms of a photoinduced SRN1 substitution. The 1,1-bis(trimethylstannyl)cyclopropanes were obtained in good to excellent isolated yield (71−90%); 7,7-dichloro-2-oxa-bicyclo[4.1.0]heptane gave the 1,1-bis(trimethylstannyl) product in only 40% yield.
表征谱图
-
氢谱1HNMR
-
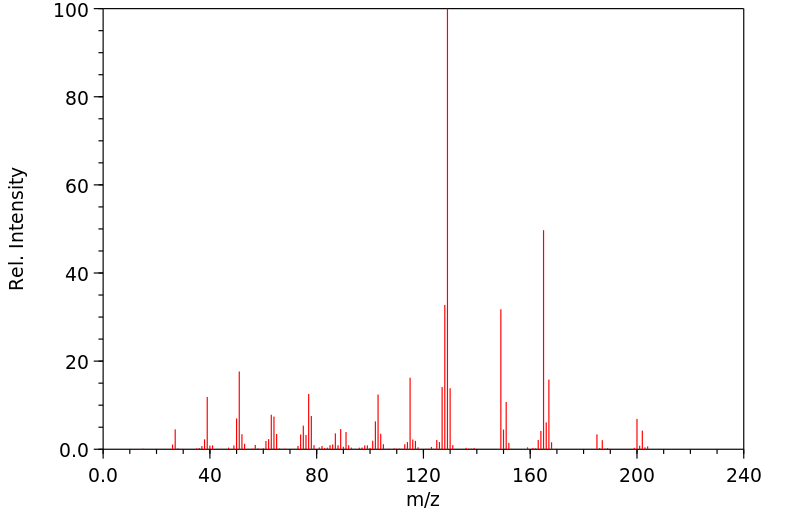
质谱MS
-
碳谱13CNMR
-
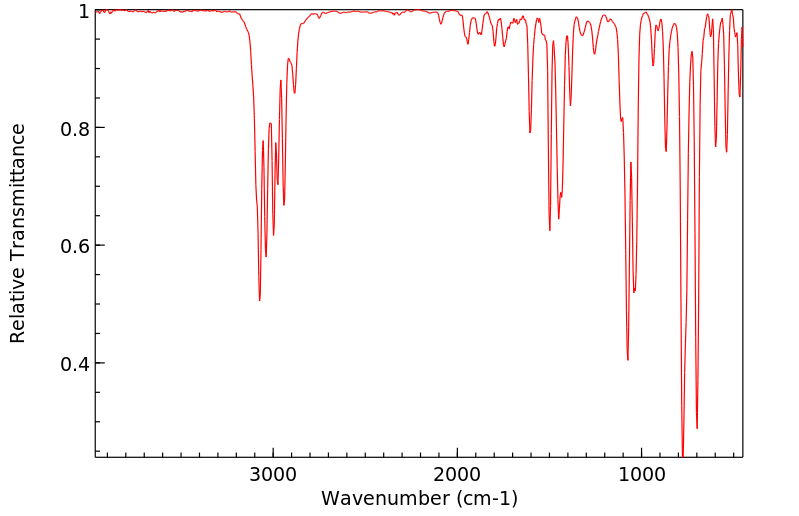
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫