乙酰基环已烯 | 932-66-1
物质功能分类
中文名称
乙酰基环已烯
中文别名
1-乙酰基-1-环己烯;1-乙酰环己烯
英文名称
1-cyclohexenyl methyl ketone
英文别名
1-Acetyl-1-cyclohexene;1-acetylcyclohexene;1-(cyclohexen-1-yl)ethanone
CAS
932-66-1
化学式
C8H12O
mdl
——
分子量
124.183
InChiKey
LTYLUDGDHUEBGX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:73 °C
-
沸点:201-202 °C(lit.)
-
密度:0.966 g/mL at 25 °C(lit.)
-
闪点:150 °F
-
LogP:1.648 (est)
-
保留指数:931
-
稳定性/保质期:
- 在常温常压下稳定,应避免与强碱和氧化物接触。
- 主要存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.62
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2914299000
-
危险性防范说明:P210,P280,P370+P378,P403+P235,P501
-
危险性描述:H227,H302
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
| Name: | 1-Acetyl-1-cyclohexene 97% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 932-66-1 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 932-66-1 | 1-Acetyl-1-cyclohexene | 97 | 213-256-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. No information available.
The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Combustible liquid and vapor.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with skin and eyes. Avoid ingestion and inhalation. Keep away from heat and flame.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 932-66-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear very slight yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 201 - 202 deg C @ 760.00mm Hg
Freezing/Melting Point: 73 deg C
Autoignition Temperature: Not available.
Flash Point: 65 deg C ( 149.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9660g/cm3
Molecular Formula: C6H9COCH3
Molecular Weight: 124.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Ignition sources.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 932-66-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Acetyl-1-cyclohexene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 932-66-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 932-66-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 932-66-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(1,4-环己二烯-1-基)乙酮 1-acetyl-1,4-cyclohexadiene 102872-23-1 C8H10O 122.167 1-(1,3-环己二烯-1-基)乙酮 1-acetyl-1,3-cyclohexadiene 53329-13-8 C8H10O 122.167 —— (Z)-cyclooct-2-enone 23202-10-0 C8H12O 124.183 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-acetyl-2-cyclohexen-1-one 15040-96-7 C8H10O2 138.166 —— 1-(cyclohexen-1-yl)-2-propen-1-one 62672-77-9 C9H12O 136.194 1-(3-甲基环己烯-1-基)乙酮 1-(3-methyl-cyclohex-1-enyl)-ethanone 60048-69-3 C9H14O 138.21 2-乙酰基-2-环己烯-1-酮 2-acetylcyclohex-2-en-1-one 52784-38-0 C8H10O2 138.166 2-溴-1-(1-环己烯-1-基)乙酮 2-bromo-1-(cyclohex-1-en-1-yl)ethanone 137994-00-4 C8H11BrO 203.079 —— 1-(α-hydroxyacetyl)cyclohexene 65311-19-5 C8H12O2 140.182 —— 1-(cyclohex-1-enyl)but-2-en-1-one 91897-80-2 C10H14O 150.221 —— 1-(1-Cyclohexenyl)-3-methyl-2-buten-1-one 74272-34-7 C11H16O 164.247 —— 1-(1-Cyclohexenyl)-3-hydroxy-1-butanone 122847-20-5 C10H16O2 168.236 —— 2-ethylidenecyclohexan-1-one 1122-25-4 C8H12O 124.183 —— 1-(3-hydroxycyclohex-1-en-1-yl)ethanone 15040-95-6 C8H12O2 140.182 —— 1-(cyclohex-1-en-1-yl)-3-(dimethylamino)propan-1-one 54924-14-0 C11H19NO 181.278 —— 1-(1-Cyclohexenyl)-3-hydroxy-3-methyl-1-butanone 122847-21-6 C11H18O2 182.263 —— 4,4,4-trifluoro-1-(1-cyclohexenyl)-1,3-butanedione 343629-60-7 C10H11F3O2 220.191 —— 2-cyclohex-1-enylpropionaldehyde 96929-98-5 C9H14O 138.21 —— (3α,4β,4aα,8aα)-4-(1-Cyclohexenylcarbonyl)-3-methyl-3,4,4a,5,6,7,8,8a-octahydronaphthalen-1(2H)-one 101183-94-2 C18H26O2 274.403 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:构象自由与限制对不对称氢化反应速率的影响:铱催化二烯酮的区域和对映选择性单氢化摘要:研究发现,不饱和羰基的构象对于铱催化氢化反应的反应性极其重要。这种反应性差异用于二烯的新型区域选择性氢化,其中(s)-顺式构型烯烃优先被氢化。在铱催化的烯烃加氢中,区域歧视推翻了取代模式中的传统反应顺序。DOI:10.1002/chem.202303406
-
作为产物:参考文献:名称:Palladium-catalyzed cross-coupling of .beta.-(methanesulfonyl)oxyenones with organostannanes摘要:beta-(Methanesulfonyl)oxy enones, derived from 1,3-diones, cross-couple with vinylstannanes in 50-80% yields when a substoichiometric amount of Pd(PPh3)4 and stoichiometric lithium bromide are used. Phenyltributylstannane affords low yields of cross-coupled product. Tetrabutyltin, tributyltin hydride, and ethynyltributyltin do not couple under the reaction conditions. The reaction is proposed to involve in situ formation of the beta-bromo enone, oxidative addition to the Pd(0) catalyst, transmetalation, and reductive elimination to afford cross-coupled products. The analogous enol phosphates undergo coupling in low yields, the major product resulting from regeneration of the 1,3-dione.DOI:10.1021/jo00004a028
-
作为试剂:描述:参考文献:名称:Lessard, Jean; Mondon, Martine; Touchard, Daniel, Canadian Journal of Chemistry, 1981, vol. 59, p. 431 - 450摘要:DOI:
文献信息
-
Fe(0)-Mediated Synthesis of Tri- and Tetra-Substituted Olefins from Carbonyls: An Environmentally Friendly Alternative to Cr(II)作者:J. R. Falck、Romain Bejot、Deb K. Barma、Anish Bandyopadhyay、Suju Joseph、Charles MioskowskiDOI:10.1021/jo061445u日期:2006.10.1carbonyls by activated polyhalides. In many instances, Fe(0) was equivalent or superior to Cr(II). Notably, Fe(0), but not Cr(II), proved compatible with a wide range of functionality, inter alia, unprotected phenol, aryl nitro, carboxylic acid, and alkyl nitrile. A surprising reversal of stereoselectivity for aldehydes versus ketones was observed using both metals. The resultant α-halo-α,β-unsaturated or α
-
Highly chemoselective palladium-catalyzed conjugate reduction of .alpha.,.beta.-unsaturated carbonyl compounds with silicon hydrides and zinc chloride cocatalyst作者:Ehud. Keinan、Noam. GreenspoonDOI:10.1021/ja00283a029日期:1986.11experiments and 'H NMR studies, a catalytic cycle is postulated in which the first step involves reversible coordination of the palladium complex to the electron-deficient olefin and oxidative addition of silicon hydride to form a hydridopalladium olefin complex. Migratory insertion of hydride into the coordinated olefin produces an intermediate palladium enolate which, via reductive elimination, collapses back由可溶性钯催化剂、氢化硅烷和氯化锌组成的三组分体系能够有效地共轭还原α、不饱和酮和醛。最佳条件组包括二苯基硅烷作为最有效的氢化物供体,任何可溶于 0 或 I1 氧化态的钯配合物,当它被膦配体稳定时,以及作为最佳路易斯酸助催化剂的 ZnCl。该反应对于范围广泛的不饱和酮和醛非常普遍,并且对于这些迈克尔受体具有高度选择性,因为在这些条件下α,-不饱和羧酸衍生物的还原非常缓慢。当双氘代二苯基硅烷用于还原不饱和酮时,氘在底物的受阻较少的面上立体选择性地引入,并在 8 位上以区域选择性的方式引入。相反,当在痕量 D2O 存在下进行还原时,氘掺入发生在 a 位。在掺入氘的实验和 1 H NMR 研究的基础上,假定催化循环,其中第一步涉及钯配合物与缺电子烯烃的可逆配位和氢化硅的氧化加成以形成氢化钯烯烃配合物。氢化物迁移插入配位的烯烃产生中间体烯醇钯,通过还原消除,它塌缩回 Pd(0) 络合物和甲硅烷基烯
-
Mild Chemoenzymatic Oxidation of Allylic <i>sec</i>-Alcohols. Application to Biocatalytic Stereoselective Redox Isomerizations作者:Lía Martínez-Montero、Vicente Gotor、Vicente Gotor-Fernández、Iván LavanderaDOI:10.1021/acscatal.7b03293日期:2018.3.2β-unsaturated ketones. Afterward, these compounds react with different commercially available ene-reductases to afford the corresponding saturated ketones. Remarkably, in the case of trisubstituted alkenes, the bioreduction reaction occurred with high stereoselectivity. Overall, a bienzymatic one-pot two-step sequential strategy has been described with respect to the synthesis of saturated ketones starting在温和的反应条件下,在水性介质中设计催化氧化方法,并使用分子氧作为最终电子受体,是传统氧化转化的合适替代方法。如果在同一分子内存在其他可氧化的官能团(如烯丙醇的情况),则这些方法尤为重要。本文我们应用漆酶组成的简单化学酶促系统从云芝和2,2,6,6-四甲基自由基(TEMPO),以氧化一系列外消旋烯丙基的秒-醇转化为相应的α,β-不饱和酮。然后,这些化合物与不同的市售烯还原酶反应,得到相应的饱和酮。显着地,在三取代的烯烃的情况下,生物还原反应以高的立体选择性发生。总的来说,关于从外消旋烯丙基醇开始的饱和酮的合成,已经描述了双酶一锅两步顺序策略,因此类似于先前在文献中报道的这些衍生物的金属催化的氧化还原异构化。
-
Copper-Mediated Cross-Dehydrogenative Coupling of 2-Methylpyridine and 8-Methylquinoline with Methyl Ketones and Benzamides作者:Gadde Sathish Kumar、Joshua William Boyle、Ciputra Tejo、Philip Wai Hong ChanDOI:10.1002/asia.201501096日期:2016.2synthetic method to prepare (E)‐(pyridin‐2‐yl)enones and (E)‐(quinolin‐8‐yl)enones that relies on the respective copper(I)‐catalyzed formal cross‐dehydrogenative coupling (CDC) reaction of 2‐methylpyridine and 8‐methylquinoline with methyl ketones has been discovered. The mechanism was delineated to follow a pathway involving oxidation of the N‐heterocycle to its corresponding aldehyde adduct prior
-
<scp>Regio‐Divergent</scp> C—H Alkynylation with Janus Directing Strategy <i>via</i> Ir( <scp>III</scp> ) Catalysis作者:Xianwei Li、Guangxin Liang、Zhang‐Jie ShiDOI:10.1002/cjoc.202000204日期:2020.9Directing strategy has been extensively exploited to maintain activity and selectivity for the rapid access to functionalized molecules and pharmaceutical targets. However, ‘one‐to‐one’ activation model was usually achieved through traditional directing strategy. Herein, we achieved ‘one‐to‐two’ activation model by slight modification of simple and practical ketoxime and amide functionality. With judicious指导策略已得到广泛利用,以保持活性和选择性,以快速获得功能化分子和药物靶标。但是,“一对一”激活模型通常是通过传统的指导策略来实现的。在这里,我们通过对简单而实用的酮肟和酰胺功能进行一些修改,实现了“一对二”激活模型。通过明智地选择导向基团,实现了Csp 3 -H和Csp 2 -H键的炔基化反应,更重要的是,实现了脱氢的Csp 3 -H炔基化,从而实现了药物在区域上的分散后期修饰。
表征谱图
-
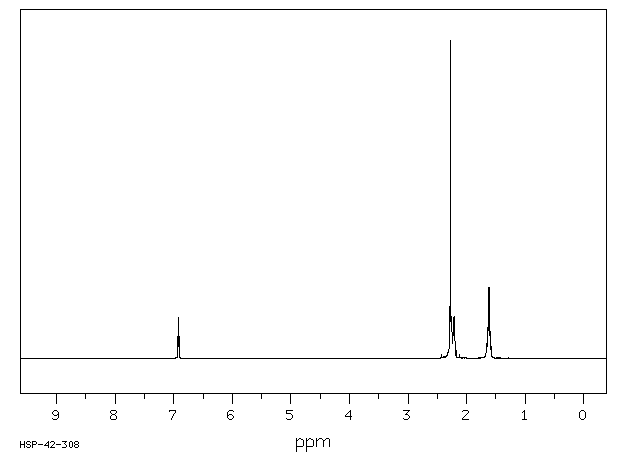
氢谱1HNMR
-
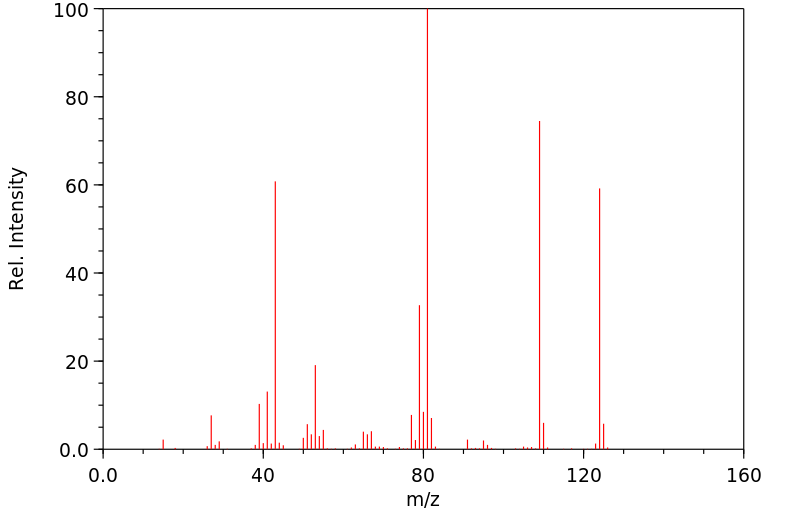
质谱MS
-
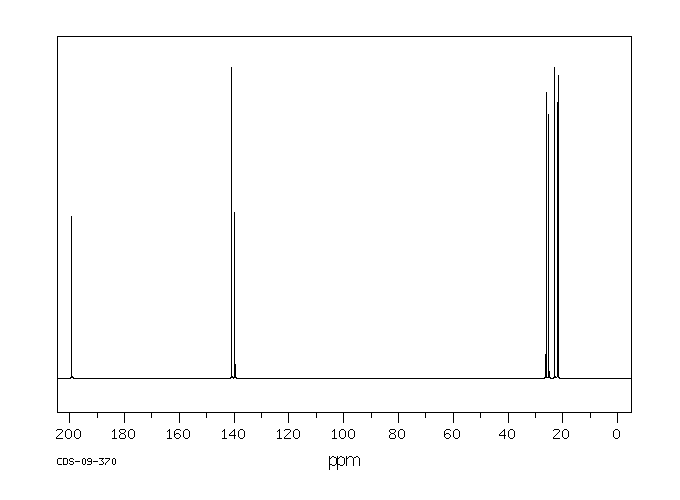
碳谱13CNMR
-
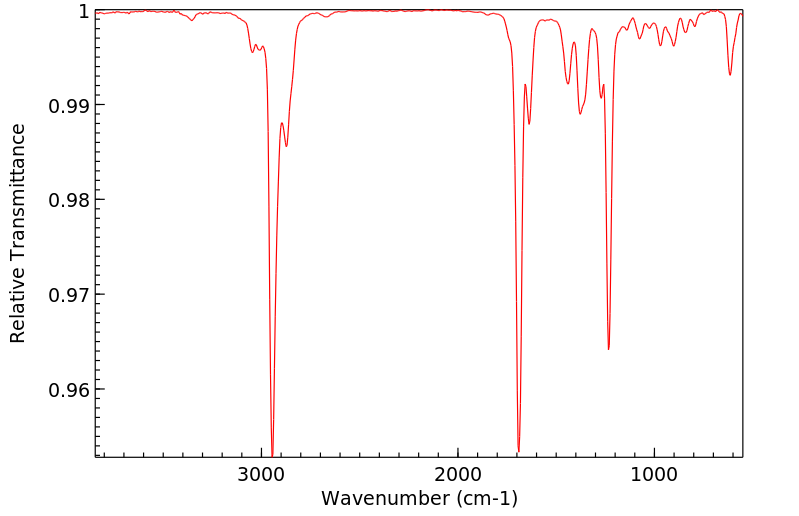
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷