4-甲氧基-1-萘酚 | 84-85-5
中文名称
4-甲氧基-1-萘酚
中文别名
1-羟基-4-甲氧基萘
英文名称
4-methoxynaphth-1-ol
英文别名
4-methoxynaphthalen-1-ol;4-methoxynaphthol;4-methoxy-α-naphthol;1-hydroxy-4-methoxynaphthalene;4-methoxy-1-naphtol;4-Methoxy-1-naphthol
CAS
84-85-5
化学式
C11H10O2
mdl
MFCD00003976
分子量
174.199
InChiKey
BOTGCZBEERTTDQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:126-129 °C (lit.)
-
沸点:265.17°C (rough estimate)
-
密度:1.0844 (rough estimate)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2909500000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:本品应密封避光保存。
SDS
1.1 产品标识符
: 4-Methoxy-1-naphthol
化学品俗名或商品名
1.2 鉴别的其他方法
1-Hydroxy-4-methoxynaphthalene
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 1-Hydroxy-4-methoxynaphthalene
别名
: C11H10O2
分子式
: 174.2 g/mol
分子量
成分 浓度
4-Methoxy-1-naphthol
-
化学文摘编号(CAS No.) 84-85-5
EC-编号 201-566-3
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 紫色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 126 - 129 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 4-Methoxy-1-naphthol
化学品俗名或商品名
1.2 鉴别的其他方法
1-Hydroxy-4-methoxynaphthalene
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 1-Hydroxy-4-methoxynaphthalene
别名
: C11H10O2
分子式
: 174.2 g/mol
分子量
成分 浓度
4-Methoxy-1-naphthol
-
化学文摘编号(CAS No.) 84-85-5
EC-编号 201-566-3
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 紫色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 126 - 129 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
制备方法
用于有机合成。
用途简介 用途用于有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-甲氧基萘 1-Methoxynaphthalene 2216-69-5 C11H10O 158.2 —— 4-(2-Chlorethoxy)-1-naphthol 73661-04-8 C12H11ClO2 222.671 —— 4-(2-Bromethoxy)-1-naphthol 99893-90-0 C12H11BrO2 267.122 —— 1-(2-Chlorethoxy)-4-methoxynaphthalin 99893-99-9 C13H13ClO2 236.698 1-(2-溴乙氧基)-4-甲氧基萘 1-(2-Bromethoxy)-4-methoxynaphthalin 99894-02-7 C13H13BrO2 281.149 1,4-二羟基萘 1,4-Dihydroxynaphthalene 571-60-8 C10H8O2 160.172 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,4-二甲氧基萘 1,4-dimethoxynaphthalene 10075-62-4 C12H12O2 188.226 1-甲氧基萘 1-Methoxynaphthalene 2216-69-5 C11H10O 158.2 —— 4-ethoxy-1-naphthol 27294-38-8 C12H12O2 188.226 —— 1-methoxy-4-allyloxynaphthalene 107777-17-3 C14H14O2 214.264 1-(2-溴乙氧基)-4-甲氧基萘 1-(2-Bromethoxy)-4-methoxynaphthalin 99894-02-7 C13H13BrO2 281.149 1-甲氧基萘-4-乙酸酯 4-methoxynaphthalen-1-yl acetate 68716-07-4 C13H12O3 216.236 2-[[(4-甲氧基-1-萘基)氧基]甲基]环氧乙烷 1-Methoxy-4-(2,3-epoxy)propoxynaphthalene 14133-78-9 C14H14O3 230.263 —— 1-(benzyloxy)-4-methoxynaphthalene 103526-26-7 C18H16O2 264.324 4-甲氧基-2-甲基-1-萘酚 4-methoxy-2-methyl-1-naphthol 3526-73-6 C12H12O2 188.226 4-甲氧基-1-萘胺 1-amino-4-methoxynaphthalene 16430-99-2 C11H11NO 173.214 —— 4-hydroxypropranolol 10476-53-6 C16H21NO3 275.348 4-甲氧基普萘洛尔 4-Methoxypropranolol 18507-09-0 C17H23NO3 289.375 —— 4-methoxynaphthalen-1-yl pivalate 1072840-83-5 C16H18O3 258.317 —— 4-methoxy-1-naphthyl hydrogen succinate 130252-20-9 C15H14O5 274.273 —— 4-methoxy-1-naphthyl hydrogen glutarate 1282527-69-8 C16H16O5 288.3 —— Tert-butyl (4-methoxynaphthalen-1-yl) carbonate 421555-84-2 C16H18O4 274.317 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:硅胶负载的硝酸铈铵 (CAN):一种简单有效的固体负载试剂,用于将含氧芳香族化合物氧化成醌类摘要:摘要 已开发出一种硅胶负载的多相硝酸铈铵 (CAN) 试剂,用于在非水介质中将含氧芳烃氧化成醌。该方法的优点包括收率高、反应条件温和、非水介质、反应时间短和易于分离产物。DOI:10.1080/00397910600616859
-
作为产物:描述:参考文献:名称:孤基热稳定细胞色素P450 CYP175A1催化取代基在萘衍生物反应中的反应性和产物选择性上的作用摘要:CYP175A1酶的热稳定性质对于在环境温度或环境良性条件下的室温或高温下的生物催化具有潜在的意义。尽管对该酶的底物选择性知之甚少,但已通过氧化途径研究了CYP175A1对不同取代萘的生物催化活性,并确定了取代基对反应的影响。该酶首先充当过氧合酶,以将这些取代的萘转化为相应的萘酚,随后将其原位进行CYP175A1的过氧化物酶型活性可能导致氧化二聚作用形成不同颜色的染料。产物分析和动力学测量表明,基质中电子释放取代基(ERS)的存在增强了基质的转化率,而基质中吸电子取代基(EWS)的存在则大大降低了基质的转化率。还发现ERS在底物中的位置在底物的转化中起重要作用。结果进一步证明Leu80残基突变为Phe通过促进活性位点中的底物缔合而增强了酶的反应性。观察到的取代萘的酶促氧化反应速率遵循取代基的哈米特相关性,DOI:10.1016/j.bioorg.2015.08.003
-
作为试剂:描述:4-甲基-联苯-2-胺 在 4-甲氧基-1-萘酚 、 C56H50Br4Fe2N4O6 、 sodium cyanoborohydride 、 三乙胺 、 三氯氧磷 作用下, 以 二氯甲烷 、 溶剂黄146 、 甲苯 为溶剂, 反应 37.0h, 生成 (R)-6-(4-methoxyphenyl)-8-methyl-5,6-dihydrophenanthridine参考文献:名称:环状仲胺的铁催化需氧脱氢动力学拆分摘要:已经报道了使用空气作为氧化剂的环状仲胺的非酶促铁催化脱氢动力学拆分。该方法经济实用,适用于5,6-二氢菲啶和1,2-二氢喹啉等一系列α位官能团多样的环状苄胺,收率高,对映选择性好。还证明了使用现有催化不对称合成策略难以获得的生物活性分子的高级中间体的直接脱氢动力学拆分。DOI:10.1021/jacs.9b00615
文献信息
-
CHLORO-PYRAZINE CARBOXAMIDE DERIVATIVES WITH EPITHELIAL SODIUM CHANNEL BLOCKING ACTIVITY申请人:Parion Sciences, Inc.公开号:US20140171447A1公开(公告)日:2014-06-19This invention provides compounds of the formula I: and their pharmaceutically acceptable salts, useful as sodium channel blockers, compositions containing the same, therapeutic methods and uses for the same and processes for preparing the same.这项发明提供了式I的化合物及其药用盐,可用作钠通道阻滞剂,包含这些化合物的组合物,以及用于这些化合物的治疗方法和用途,以及制备这些化合物的方法。
-
PHOTOSENSITIVE RESIN COMPOSITION, OXIME SULFONATE COMPOUND, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE, AND LIQUID CRYSTAL DISPLAY DEVICE申请人:FUJIFILM Corporation公开号:US20130171415A1公开(公告)日:2013-07-04Disclosed is a photosensitive resin composition comprising: (Component A) an oxime sulfonate compound represented by Formula (1); (Component B) a resin comprising a constituent unit having an acid-decomposable group that is decomposed by an acid to form a carboxyl group or a phenolic hydroxy group; and (Component C) a solvent wherein in Formula (1) R 1 denotes an alkyl group, an aryl group, or a heteroaryl group, each R 2 independently denotes a hydrogen atom, an alkyl group, an aryl group, or a halogen atom, Ar 1 denotes an o-arylene group or an o-heteroarylene group, X denotes O or S, and n denotes 1 or 2, provided that of two or more R 2 s present in the compound, at least one denotes an alkyl group, an aryl group, or a halogen atom.
-
Titanium(IV) Chloride-Mediated Ortho-Acylation of Phenols and Naphthols作者:Nurulain T. Zaveri、Ahlem BensariDOI:10.1055/s-2003-36822日期:——The use of titanium(IV) chloride as a Lewis acid for direct ortho-acylation of phenols and naphthols proves to be a convenient, more general and direct route to various hydroxyaryl ketones. The route is regioselective, leading to ortho C-acylated products in satisfactory to high yields in most cases.
-
Naphthylamino-and naphthyloxy-pyridinamine comounds useful as topical申请人:Hoechst-Roussel Pharmaceuticals Inc.公开号:US04931457A1公开(公告)日:1990-06-05There are described compounds of the formula ##STR1## where R is hydrogen, loweralkyl, arylloweralkyl or loweralkylcarbonyl; X is O or NR.sub.1, R.sub.1 being hydrogen, loweralkyl or loweralkylcarbonyl; and Y is hydrogen, loweralkyl, loweralkoxy, halogen or trifluoromethyl; which compounds are useful as topical antiinflammatory agents for the treatment of skin disorders.描述了化合物的公式##STR1##,其中R是氢、较低的烷基、芳基较低的烷基或较低的烷基羰基;X是O或NR.sub.1,R.sub.1是氢、较低的烷基或较低的烷基羰基;Y是氢、较低的烷基、较低的烷氧基、卤素或三氟甲基;这些化合物可用作治疗皮肤疾病的局部抗炎药。
-
Convenient synthesis of Bioactive Pyranonaphthoquinones (±)9-Demethoxyeleutherin and (±)9-Demethoxyisoeleutherin作者:Rohan A. Limaye、Augustine R. Joseph、Arun D. Natu、Madhusudan V. ParadkarDOI:10.3184/174751914x14108836482583日期:2014.9
A convenient synthesis of (±)9-demethoxyeleutherin and (±)9-demethoxyisoeleutherin, the analogues of naturally occurring pyranonaphthoquinone antibiotics eleutherin and isoeleutherin, is described. The synthesis was achieved from 4-methoxy-1-napthol in four simple steps including Claisen rearrangement and oxymercuration–demercuration reactions.
描述了天然存在的吡喃萘醌类抗生素 eleutherin 和 isoeleutherin 的类似物 (±)9-demethoxyeleutherin 和 (±)9-demethoxyisoeleutherin 的简便合成方法。合成过程由 4-甲氧基-1-萘酚通过四个简单步骤完成,包括克莱森重排和氧巯基-脱巯基反应。
表征谱图
-
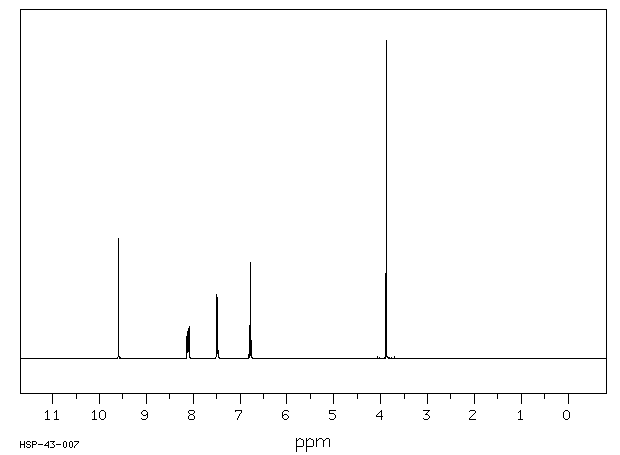
氢谱1HNMR
-
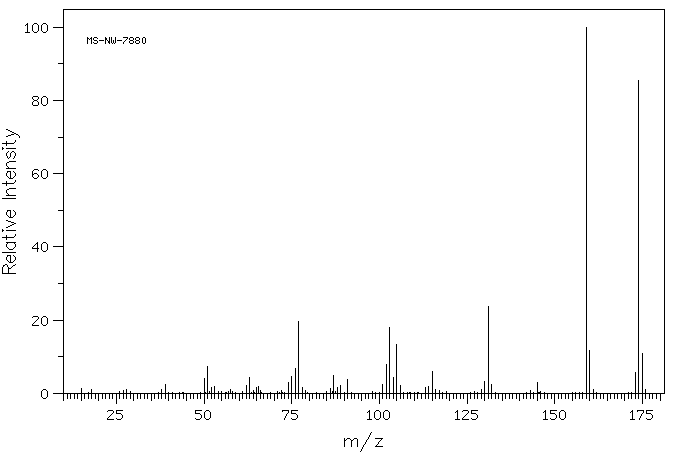
质谱MS
-
碳谱13CNMR
-
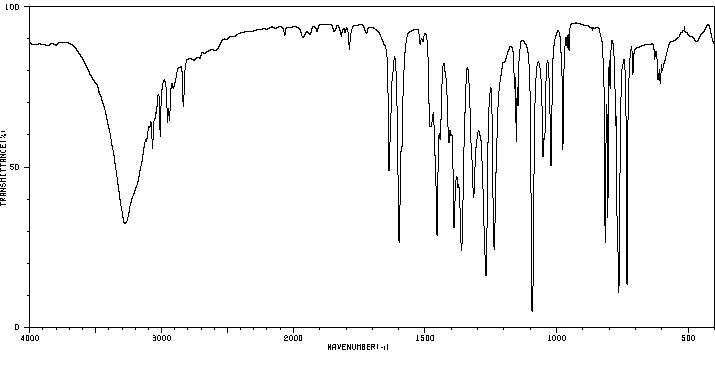
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮