3-Methoxycarbonylpyrazine 1-oxide | 770-00-3
中文名称
——
中文别名
——
英文名称
3-Methoxycarbonylpyrazine 1-oxide
英文别名
Methyl 2-pyrazinecarboxylate 4-oxide;methyl 4-oxidopyrazin-4-ium-2-carboxylate
CAS
770-00-3
化学式
C6H6N2O3
mdl
MFCD00233244
分子量
154.125
InChiKey
ZTAQCRRTNOJVIU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172-173 °C
-
沸点:408.7±25.0 °C(Predicted)
-
密度:1.32±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-1
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:64.6
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-吡嗪羧酸4-氧化物 3-carboxypyrazine 1-oxide 874-54-4 C5H4N2O3 140.098 吡嗪-2-羧酸甲酯 methyl pyrazine-2-carboxylate 6164-79-0 C6H6N2O2 138.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 吡嗪-2-羧酸甲酯 methyl pyrazine-2-carboxylate 6164-79-0 C6H6N2O2 138.126 5-氯吡嗪-2-羧酸甲酯 methyl 5-chloropyrazine-2-carboxylate 33332-25-1 C6H5ClN2O2 172.571 甲基5-硫代-4,5-二氢-2-吡嗪羧酸酯 methyl 5-mercaptopyrazine-2-carboxylate 147032-26-6 C6H6N2O2S 170.192 甲基5-氰基-2-吡嗪羧酸酯 Methyl 5-cyanopyrazinecarboxylate 138560-54-0 C7H5N3O2 163.136 6-氯-2-吡嗪甲酸甲酯 6-chloro-pyrazine-2-carboxylic acid methyl ester 23611-75-8 C6H5ClN2O2 172.571 3-氯-2-吡嗪甲酸甲酯 methyl 3-chloropyrazine-2-carboxylate 27825-21-4 C6H5ClN2O2 172.571 —— methyl 3-cyanopyrazine-2-carboxylate 74402-57-6 C7H5N3O2 163.136
反应信息
-
作为反应物:描述:参考文献:名称:Structure–Activity Studies of Diazabicyclo[3.3.0]octane-Substituted Pyrazines and Pyridines as Potent α4β2 Nicotinic Acetylcholine Receptor Ligands摘要:A series of diazabicyclo[3.3.0]octane substituted pyridines and pyrazines was synthesized and characterized at the alpha 4 beta 2 neuronal nicotinic acetylcholine receptor (nAChR). The compounds were designed to mimic the profile of ABT-089, high affinity binding ligand for the alpha 4 beta 2 nAChR, with limited agonist activity. Carboxamide derivatives of 3-(diazabicyclo[3.3.0]octane)-substituted pyridines or 2-(diazabicyclo[3.3.0]octane)-substituted pyrazines were found to have the desired binding and activity profile. The structure-activity relationship of these compounds is presented.DOI:10.1021/jm201045m
-
作为产物:描述:参考文献:名称:Ambrogi; Cozzi; Sanjust, European Journal of Medicinal Chemistry, 1980, vol. 15, # 2, p. 157 - 163摘要:DOI:
文献信息
-
Highly chemoselective deoxygenation of N-heterocyclic <i>N</i>-oxides under transition metal-free conditions作者:Se Hyun Kim、Ju Hyeon An、Jun Hee LeeDOI:10.1039/d1ob00260k日期:——the most useful tools for synthesizing various N-heterocyclic compounds, the highly chemoselective deoxygenation of densely functionalized N-heterocyclic N-oxides has received much attention from the synthetic chemistry community. Here, we provide a protocol for the highly chemoselective deoxygenation of various functionalized N-oxides under visible light-mediated photoredox conditions with Na2-eosin
-
INHIBITORS OF COLLAGEN PROLYL 4-HYDROXYLASE申请人:WISCONSIN ALUMNI RESEARCH FOUNDATION公开号:US20160280701A1公开(公告)日:2016-09-29Biheteroaryl dicarboxylates and esters, and salts thereof which are useful as modulators of CP4H activity and more particularly as inhibitors of CP4H. Compounds of formula: and salts thereof where: X is S, O, NH, or NR, where R is an alkyl group having 1-3 carbon atoms; R 1 and R 2 independently are —OR 7 , or —NHSO 2 R 8 , where R 7 is selected from: hydrogen, alkyl, alkenyl, alkoxyalkyl, —R′—CO—R″, —R′—CO—O—R″, —CO—R″, —R′—O—CO—R″, —R′—CO—NR″, —CO—NR″, or —R′—O—CO—NR″, and R 8 is selected from hydrogen, alkyl, aryl, arylalkyl; R 3 , R 4 and R 6 independently are hydrogen, alkyl, alkoxy, alkenyl, alkenoxy, halo alkyl, haloalkenyl, halogen, hydroxyl, hydroxyalkyl, hydroxyalkenyl, aryl, aryloxy, arylalkyl or arylalkyloxy; R 5 is hydrogen, halogen, alkyl having 1-3 carbon atoms, or alkoxy having 1-3 carbon atoms; —R′— is a divalent straight chain or branched alkylene, and —R″ is an alkyl, alkenyl, arylalkyl, or aryl group. Methods for inhibition of CP4H in vivo and in vitro.Biheteroaryl二羧酸酯和酯,以及它们的盐,可用作CP4H活性的调节剂,更具体地作为CP4H的抑制剂。化合物的结构式:及其盐,其中:X为S、O、NH或NR,其中R为具有1-3个碳原子的烷基基团;R1和R2独立地为—OR7,或—NHSO2R8,其中R7选自:氢、烷基、烯基、烷氧基烷基、—R′—CO—R″、—R′—CO—O—R″、—CO—R″、—R′—O—CO—R″、—R′—CO—NR″、—CO—NR″或—R′—O—CO—NR″,而R8选自氢、烷基、芳基、芳基烷基;R3、R4和R6独立地为氢、烷基、烷氧基、烯基、烯氧基、卤代烷基、卤代烯基、卤素、羟基、羟基烷基、羟基烯基、芳基、芳氧基、芳基烷基或芳基烷氧基;R5为氢、卤素、具有1-3个碳原子的烷基,或具有1-3个碳原子的烷氧基;—R′—为二价的直链或支链烷基,而—R″为烷基、烯基、芳基烷基或芳基。用于体内和体外抑制CP4H的方法。
-
Studies on Pyrazines; 21.<sup>1</sup>A Convenient Synthesis of Pyrazinecarboxylic Acid Derivatives from Chloropyrazines作者:Ryo Takeuchi、Katsunobu Suzuki、Nobuhiro SatoDOI:10.1055/s-1990-27055日期:——Palladium-catalyzed carbonylation of chloropyrazines in methanol led to pyrazinecarboxylic esters in good yields. Similarly, pyrazinecarboxamides were obtained by using dialkylamines or alkylamines as the solvent.
-
Selective Ligands for the Neuronal Nicotinic Receptors and Uses Thereof申请人:Bunnelle William H.公开号:US20090281118A1公开(公告)日:2009-11-12The present application describes selective ligands of formula (I) for neuronal nicotinic receptors (NNRs), more specifically for the α4β2 NNR subtype, compositions thereof, and methods of using the same, wherein X, R 1 , X, R 2 , R 3 , L 1 , m, n, p, and q are defined in the specification.
-
SELECTIVE SUBSTITUTED PYRIDINE LIGANDS FOR NEURONAL NICOTINIC RECEPTORS申请人:Bunnelle William H.公开号:US20120190692A1公开(公告)日:2012-07-26The present application describes selective ligands of formula (I) for neuronal nicotinic receptors (NNRs), more specifically for the α4β2 NNR subtype, compositions thereof, and methods of using the same, wherein X, R 1 , X, R 2 , R 3 , L 1 , m, n, p, and q are defined in the specification.
表征谱图
-
氢谱1HNMR
-
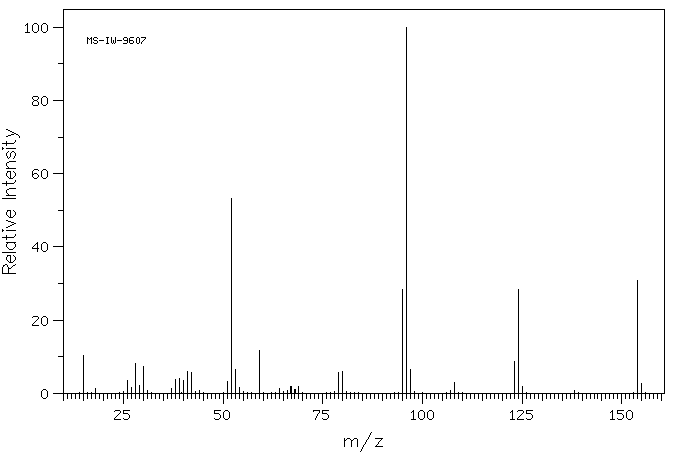
质谱MS
-
碳谱13CNMR
-
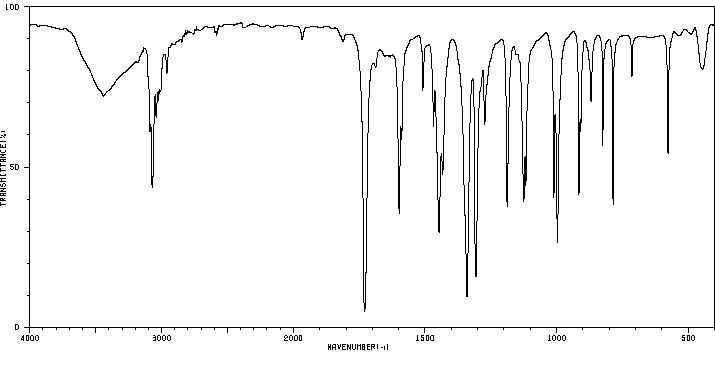
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3