6H-吲哚并[2,3-b]喹喔啉 | 243-59-4
中文名称
6H-吲哚并[2,3-b]喹喔啉
中文别名
——
英文名称
indophenazine
英文别名
6H-indolo[2,3-b]quinoxaline;indolo<2,3-b>quinoxaline;6H-Indolo<2,3-b>chinoxalin;Indolo<2,3-b>chinoxalin;6H-indolo [3,2-b]quinoxaline;6H-indolo[3,2-b]quinoxaline
CAS
243-59-4
化学式
C14H9N3
mdl
MFCD00068754
分子量
219.246
InChiKey
LCKIWLDOHFUYDV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:430.5±28.0 °C(Predicted)
-
密度:1.38±0.1 g/cm3(Predicted)
-
熔点:295-297 °C
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:17
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:41.6
-
氢给体数:1
-
氢受体数:2
安全信息
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 6-acetyl-6H-indolo[2,3-b]quinoxaline 13860-54-3 C16H11N3O 261.283 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 9-bromo-6H-indolo[2,3-b]quinoxaline 57743-36-9 C14H8BrN3 298.142 —— 9-nitro-6H-indolo[2,3-b]quinoxaline 57743-37-0 C14H8N4O2 264.243 —— 1-N-methyl-indolo <2,3-b> quinoxaline 65880-39-9 C15H11N3 233.272 —— 6-allyl-6H-indolo[2,3-b]quinoxaline 304003-29-0 C17H13N3 259.31 —— 6-(prop-2-yn-1-yl)-6H-indolo[2,3-b]quinoxaline 388109-75-9 C17H11N3 257.294 —— 6-(2-bromoethyl)-6H-indolo[2,3-b]quinoxaline 612051-61-3 C16H12BrN3 326.195 —— 6-butyl-6H-indolo[2,3-b]quinoxaline 327061-52-9 C18H17N3 275.353 —— 6-(3-chloropropyl)-6H-indolo[2,3-b]quinoxaline 1082392-57-1 C17H14ClN3 295.771 6-(2-二甲基氨基乙基)-6H-吲哚并(2,3-b)-喹喔啉 6-(2-dimethylaminoethyl)-6H-indolo<2,3-b>-quinoxaline 25681-09-8 C18H18N4 290.368 —— 6-benzyl-6H-indolo[2,3-b]quinoxaline —— C21H15N3 309.37 —— 6-[2-(diethylamino)ethyl]-6H-indolo[2,3-b]quinoxaline 59184-53-1 C20H22N4 318.421 —— 2-(3-(6H-indolo[2,3-b]quinoxalin-6-yl)propylamino)ethanol 1216746-12-1 C19H20N4O 320.394 —— (S)-5-(indolo[2,3-b]quinoxalin-6-yl)-pentane-1,2-diol 917076-94-9 C19H19N3O2 321.379 —— (S)-4-(indolo[2,3-b]quinoxalin-6-yl)-butane-1,2-diol 917076-93-8 C18H17N3O2 307.352 —— 6-(3-diethylamino)propyl-6H-indolo[2,3-b]quinoxaline 1215369-46-2 C21H24N4 332.448 吲哚并[2,3-b]喹噁啉-6-乙酸 indolo[2,3-b]quinoxalin-6-yl-acetic acid 25681-06-5 C16H11N3O2 277.282 —— 6-phenyl-6H-indolo[2,3-b]quinoxaline 693794-96-6 C20H13N3 295.343 —— 6-(3-(piperidin-1-yl))propyl-6H-indolo[2,3-b]quinoxaline 111756-97-9 C22H24N4 344.459 —— 6-(2-morpholin-4-ylethyl)-6H-indolo[2,3-b]quinoxaline 172038-68-5 C20H20N4O 332.405 —— (S)-3-(indolo[2,3-b]quinoxalin-6-yl)-propane-1,2-diol 917076-91-6 C17H15N3O2 293.325 —— 6-acetyl-6H-indolo[2,3-b]quinoxaline 13860-54-3 C16H11N3O 261.283 —— Methyl 2-indolo[3,2-b]quinoxalin-6-ylacetate 443328-91-4 C17H13N3O2 291.309 —— 6-(hydrazinocarbonylmethyl)-6H-indolo[2,3-b]quinoxaline 116989-58-3 C16H13N5O 291.312 —— ethyl 2-(6H-indolo[2,3-b]quinoxalin-6-yl)acetate 25681-03-2 C18H15N3O2 305.336 —— 9-bromo-6-butyl-6H-indolo[2,3-b]quinoxaline 327061-54-1 C18H16BrN3 354.249 - 1
- 2
- 3
反应信息
-
作为反应物:描述:6H-吲哚并[2,3-b]喹喔啉 在 N-溴代丁二酰亚胺(NBS) 、 苄基三乙基氯化铵 、 sodium hydroxide 作用下, 以 水 、 N,N-二甲基甲酰胺 、 苯 为溶剂, 反应 36.0h, 生成 9-bromo-6-butyl-6H-indolo[2,3-b]quinoxaline参考文献:名称:Synthesis, Spectra, and Theoretical Investigations of the Triarylamines Based on 6H-Indolo[2,3-b]quinoxaline摘要:Triarylamines containing a 6H-indolo[2,3-b]quinoxaline core and aromatic units such as phenyl, naphthyl, pyrene, anthracene, or fluorene have been synthesized by employing palladium-catalyzed C-N and C-C coupling reactions and characterized by optical absorption and emission spectra, electrochemical behavior, and thermal studies. Even though the electronic absorption spectra of the compounds were influenced by the nature of the peripheral amines, the emission spectra indicated close similarity for the excited states in these compounds. For the derivatives in which the amines were directly anchored on the 6H-indolo[2,3-b]quinoxaline nucleus, the emission appeared to be dominated by the state localized on the 6H-indolo[2,3-b]quinoxaline chromophore, while in the compounds containing the extended conjugation the fluorescence originated from the polyaromatic linker. The compounds displayed green or yellow emission depending on the nature of the amine segment. All of the dyes displayed one-electron quasi-reversible oxidation couple in the cyclic voltammograms, which is attributable to the oxidation of the peripheral amines at the 6H-indolo[2,3-b]-quinoxaline core. An additional one-electron oxidation process observable at the high positive potentials for the compounds 7 and 8 probably arises from the oxidation of the arylthiophene segment. The enhanced thermal stability and relatively higher glass transition temperatures observed for these compounds were attributed to the presence of dipolar 6H-indolo[2,3-b]quinox. aline segment. The origin of the optical spectra and the trends observed therein were rationalized using TDDFT simulations.DOI:10.1021/jo1016663
-
作为产物:描述:N-乙酰基-3-吲哚啉酮 在 氢氧化钾 、 溴 、 四氯苯醌 作用下, 以 1,4-二氧六环 、 甲醇 、 乙醚 、 二氯甲烷 、 甲苯 为溶剂, 反应 74.33h, 生成 6H-吲哚并[2,3-b]喹喔啉参考文献:名称:A Straightforward Synthesis of Pyridopyrazino[2,3-b]indoles and Indolo[2,3-b]quinoxaline摘要:DOI:10.3987/com-01-9181
文献信息
-
One-Pot Construction of Indolo[2,3-<i>b</i>]quinoxalines through Ruthenium-Catalyzed <i>Ortho</i> C–H Bond Functionalization of 2-Arylquinoxalines with Sulfonyl Azides作者:Sudip Laru、Suvam Bhattacharjee、Sumit Ghosh、Alakananda HajraDOI:10.1021/acs.orglett.1c02837日期:2021.10.1N-substituted indolo[2,3-b]quinoxalines has been developed through a Ru(II)-catalyzed ortho C–H functionalization of 2-arylquinoxalines with sulfonyl azides and further oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in one pot. This double C–N bond formation strategy provides a new efficient route for the preparation of a series of biologically relevant 6H-indolo[2,3-b]quinoxaline derivatives in
-
Microwave-assisted Synthesis of 6-{(5-Aryl-1,3,4-oxadiazol-2-yl)methyl}-6<i>H</i>-indolo[2,3-<i>b</i>]quinoxalines作者:Sreenivas Avula、Jayaram Reddy Komsani、Satish Koppireddi、Rambabu YadlaDOI:10.1002/jhet.2272日期:2015.11convenient synthesis of a new series of 2‐(6H‐indolo[2,3‐b]quinoxalin‐6‐yl)methyl}‐5‐aryl‐1,3,4‐oxadiazoles from readily available 1,2‐diaminobenzene and isatins under microwave irradiation conditions was disclosed. The 6‐(5‐aryl‐1,3,4‐oxadiazol‐2‐yl)methyl}‐6H‐indolo[2,3‐b]quinoxalines were also prepared by the thermal cyclo‐condensation reaction of 2‐(6H‐indolo[2,3‐b]quinoxalin‐6‐yl)acetohydrazides
-
Cerium oxide nanoparticle-catalyzed three-component protocol for the synthesis of highly substituted novel quinoxalin-2-amine derivatives and 3,4-dihydroquinoxalin-2-amines in water作者:Naushad Edayadulla、Yong Rok LeeDOI:10.1039/c4ra00717d日期:——The syntheses of novel quinoxalin-2-amine derivatives were conducted in water using CeO2 nanoparticle catalyzed three-component reactions of 1,2-diamines with aldehydes and isocyanides. A variety of 3,4-dihydroquinoxalin-2-amine derivatives were also synthesized by reactions between 1,2-diamines, ketones and isocyanides. This new method offers an environmentally benign and effective approach for the
-
[EN] METHODS AND COMPOSITIONS OF SUBSTITUTED 5H-[1,2,5]OXADIAZOLO[3',4':5,6] PYRAZIONO[2,3-B]INDOLE ANALOGS AS INHIBITORS OF BETA-CATENIN/T-CELL FACTOR PROTEIN-PROTEIN INTERACTIONS<br/>[FR] PROCÉDÉS ET COMPOSITIONS D'ANALOGUES SUBSTITUÉS DE 5H-[1,2,5]OXADIAZOLO[3',4':5,6] PYRAZIONO[2,3-B]INDOLE UTILISÉS COMME INHIBITEURS DES INTERACTIONS PROTÉINE-PROTÉINE BÊTA-CATÉNINE/FACTEURS TCF申请人:UNIV UTAH RES FOUND公开号:WO2016187050A1公开(公告)日:2016-11-24In one aspect, the invention relates to substituted 5H-[1,2,5]oxadiazolo [3',4':5,6]pyrazino[2,3-b]indole analogues, derivatives thereof, and related compound; synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of treating disorders, e.g., various tumors and cancers, associated with a β-catenin/T-cell factor interaction dysfunction using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
-
Virtual screening, synthesis and biological evaluation of DNA intercalating antiviral agents作者:Kyrylo Klimenko、Sergey Lyakhov、Marina Shibinskaya、Alexander Karpenko、Gilles Marcou、Dragos Horvath、Marina Zenkova、Elena Goncharova、Rinat Amirkhanov、Andrei Krysko、Sergei Andronati、Igor Levandovskiy、Pavel Polishchuk、Victor Kuz'min、Alexandre VarnekDOI:10.1016/j.bmcl.2017.06.035日期:2017.8scaffolds of known DNA intercalators. This resulted in 12 hits which then were synthesized and tested for antiviral activity against VaV together with 43 compounds earlier studied against VSV. Two compounds displaying high antiviral activity against VaV and low cytotoxicity were selected for further antiviral activity investigations.
表征谱图
-
氢谱1HNMR
-
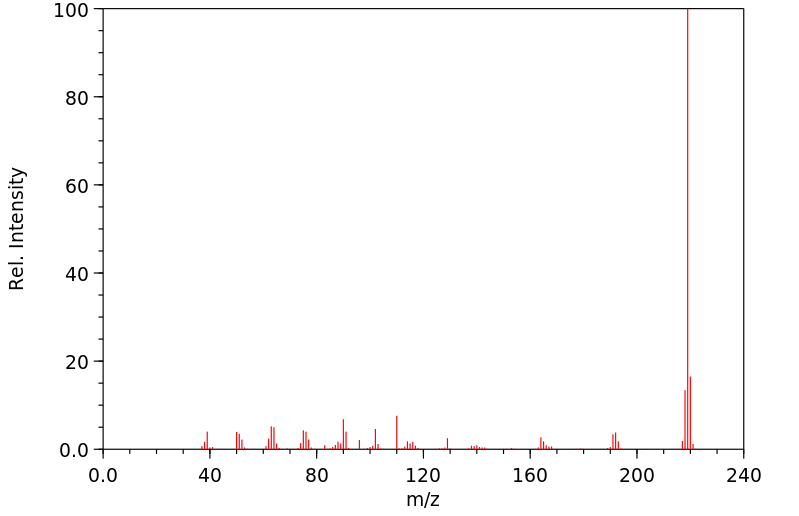
质谱MS
-
碳谱13CNMR
-
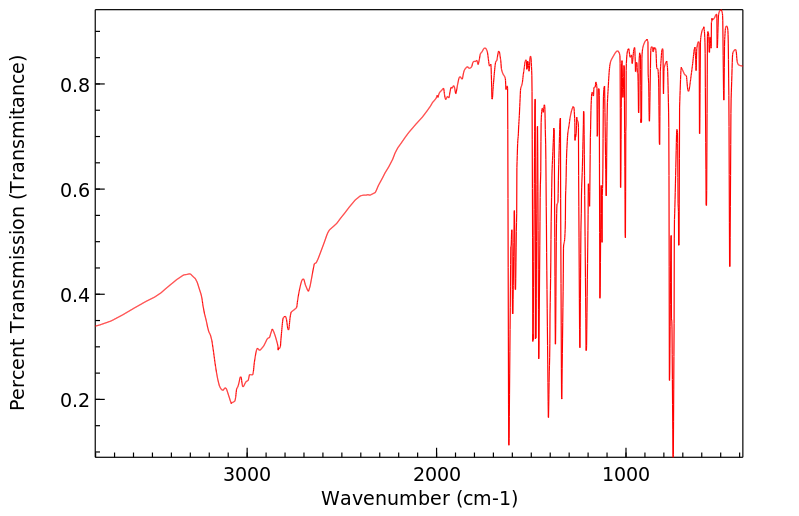
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮